Redox regulation of mitochondrial fission, protein misfolding, synaptic damage, and neuronal cell death: potential implications for Alzheimer's and Parkinson's diseases
- PMID: 20177970
- PMCID: PMC2978885
- DOI: 10.1007/s10495-010-0476-x
Redox regulation of mitochondrial fission, protein misfolding, synaptic damage, and neuronal cell death: potential implications for Alzheimer's and Parkinson's diseases
Abstract
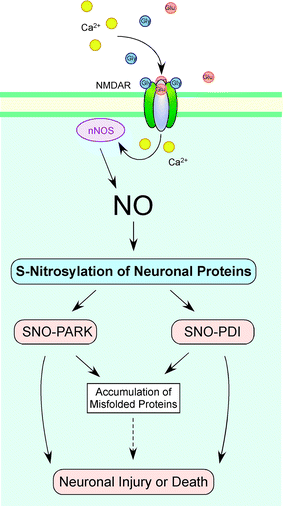
Normal mitochondrial dynamics consist of fission and fusion events giving rise to new mitochondria, a process termed mitochondrial biogenesis. However, several neurodegenerative disorders manifest aberrant mitochondrial dynamics, resulting in morphological abnormalities often associated with deficits in mitochondrial mobility and cell bioenergetics. Rarely, dysfunctional mitochondrial occur in a familial pattern due to genetic mutations, but much more commonly patients manifest sporadic forms of mitochondrial disability presumably related to a complex set of interactions of multiple genes (or their products) with environmental factors (G × E). Recent studies have shown that generation of excessive nitric oxide (NO), in part due to generation of oligomers of amyloid-β (Aβ) protein or overactivity of the NMDA-subtype of glutamate receptor, can augment mitochondrial fission, leading to frank fragmentation of the mitochondria. S-Nitrosylation, a covalent redox reaction of NO with specific protein thiol groups, represents one mechanism contributing to NO-induced mitochondrial fragmentation, bioenergetic failure, synaptic damage, and eventually neuronal apoptosis. Here, we summarize our evidence in Alzheimer's disease (AD) patients and animal models showing that NO contributes to mitochondrial fragmentation via S-nitrosylation of dynamin-related protein 1 (Drp1), a protein involved in mitochondrial fission. These findings may provide a new target for drug development in AD. Additionally, we review emerging evidence that redox reactions triggered by excessive levels of NO can contribute to protein misfolding, the hallmark of a number of neurodegenerative disorders, including AD and Parkinson's disease. For example, S-nitrosylation of parkin disrupts its E3 ubiquitin ligase activity, and thereby affects Lewy body formation and neuronal cell death.
Figures

References
-
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. - PubMed
-
- Barnham KJ, Masters CL, Bush AI. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov. 2004;3:205–214. - PubMed
-
- Muchowski PJ. Protein misfolding, amyloid formation, and neurodegeneration: a critical role for molecular chaperones? Neuron. 2002;35:9–12. - PubMed
-
- Emerit J, Edeas M, Bricaire F. Neurodegenerative diseases and oxidative stress. Biomed Pharmacother. 2004;58:39–46. - PubMed
-
- Beal MF. Experimental models of Parkinson’s disease. Nat Rev Neurosci. 2001;2:325–334. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

