Improved HIV and substance abuse treatment outcomes for released HIV-infected prisoners: the impact of buprenorphine treatment
- PMID: 20177974
- PMCID: PMC2900572
- DOI: 10.1007/s11524-010-9438-4
Improved HIV and substance abuse treatment outcomes for released HIV-infected prisoners: the impact of buprenorphine treatment
Abstract
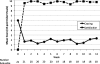
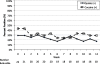
HIV-infected prisoners fare poorly after release. Though rarely available, opioid agonist therapy (OAT) may be one way to improve HIV and substance abuse treatment outcomes after release. Of the 69 HIV-infected prisoners enrolled in a randomized controlled trial of directly administered antiretroviral therapy, 48 (70%) met DSM-IV criteria for opioid dependence. Of these, 30 (62.5%) selected OAT, either as methadone (N = 7, 14.5%) or buprenorphine/naloxone (BPN/NLX; N = 23, 48.0%). Twelve-week HIV and substance abuse treatment outcomes are reported as a sub-study for those selecting BPN/NLX. Retention was high: 21 (91%) completed BPN/NLX induction and 17 (74%) remained on BPN/NLX after 12 weeks. Compared with baseline, the proportion with a non-detectable viral load (61% vs 63% log(10) copies/mL) and mean CD4 count (367 vs 344 cells/mL) was unchanged at 12 weeks. Opiate-negative urine testing remained 83% for the 21 who completed induction. Using means from 10-point Likert scales, opioid craving was reduced from 6.0 to 1.8 within 3 days of BPN/NLX induction and satisfaction remained high at 9.5 throughout the 12 weeks. Adverse events were few and mild. BPN/NLX therapy was acceptable, safe and effective for both HIV and opioid treatment outcomes among released HIV-infected prisoners. Future randomized controlled trials are needed to affirm its benefit in this highly vulnerable population.
Figures
Similar articles
-
Retention on buprenorphine is associated with high levels of maximal viral suppression among HIV-infected opioid dependent released prisoners.PLoS One. 2012;7(5):e38335. doi: 10.1371/journal.pone.0038335. Epub 2012 May 31. PLoS One. 2012. PMID: 22719814 Free PMC article.
-
HIV clinic-based buprenorphine plus naloxone versus referral for methadone maintenance therapy for treatment of opioid use disorder in HIV clinics in Vietnam (BRAVO): an open-label, randomised, non-inferiority trial.Lancet HIV. 2021 Feb;8(2):e67-e76. doi: 10.1016/S2352-3018(20)30302-7. Lancet HIV. 2021. PMID: 33539760 Free PMC article. Clinical Trial.
-
HIV treatment outcomes among HIV-infected, opioid-dependent patients receiving buprenorphine/naloxone treatment within HIV clinical care settings: results from a multisite study.J Acquir Immune Defic Syndr. 2011 Mar 1;56 Suppl 1(Suppl 1):S22-32. doi: 10.1097/QAI.0b013e318209751e. J Acquir Immune Defic Syndr. 2011. PMID: 21317590 Free PMC article. Clinical Trial.
-
The medical management of opioid dependence in HIV primary care settings.Curr HIV/AIDS Rep. 2006 Nov;3(4):195-204. doi: 10.1007/s11904-006-0016-z. Curr HIV/AIDS Rep. 2006. PMID: 17089480 Review.
-
Opioid dependence: rationale for and efficacy of existing and new treatments.Clin Infect Dis. 2006 Dec 15;43 Suppl 4:S173-7. doi: 10.1086/508180. Clin Infect Dis. 2006. PMID: 17109303 Review.
Cited by
-
Methadone dose at the time of release from prison significantly influences retention in treatment: implications from a pilot study of HIV-infected prisoners transitioning to the community in Malaysia.Drug Alcohol Depend. 2013 Sep 1;132(1-2):378-82. doi: 10.1016/j.drugalcdep.2013.01.005. Epub 2013 Feb 13. Drug Alcohol Depend. 2013. PMID: 23414931 Free PMC article.
-
Co-located Opioid Use Disorder and Hepatitis C Virus Treatment Is Not Only Right, But It Is Also the Smart Thing To Do as It Improves Outcomes!Clin Infect Dis. 2020 Oct 23;71(7):1723-1725. doi: 10.1093/cid/ciaa111. Clin Infect Dis. 2020. PMID: 32011653 Free PMC article. No abstract available.
-
Strategies for hepatitis C testing and linkage to care for vulnerable populations: point-of-care and standard HCV testing in a mobile medical clinic.J Community Health. 2014 Oct;39(5):922-34. doi: 10.1007/s10900-014-9932-9. J Community Health. 2014. PMID: 25135842 Free PMC article.
-
Maintenance on extended-release naltrexone is associated with reduced injection opioid use among justice-involved persons with opioid use disorder.J Subst Abuse Treat. 2022 Nov;142:108852. doi: 10.1016/j.jsat.2022.108852. Epub 2022 Jul 30. J Subst Abuse Treat. 2022. PMID: 35988513 Free PMC article. Clinical Trial.
-
In Their Own Voices: Breaking the Vicious Cycle of Addiction, Treatment and Criminal Justice Among People who Inject Drugs in Ukraine.Drugs (Abingdon Engl). 2016;23(2):163-175. doi: 10.3109/09687637.2015.1127327. Epub 2016 Feb 16. Drugs (Abingdon Engl). 2016. PMID: 27458326 Free PMC article.
References
-
- Spaulding AC, Seals RM, Page MJ, Brzozowski AK, Rhodes W, Hammett TM. HIV/AIDS among inmates of and releasees from US correctional facilities, 2006: declining share of epidemic but persistent public health opportunity. PLoS ONE. 2009;4(11):e7558. doi: 10.1371/journal.pone.0007558. - DOI - PMC - PubMed
-
- Centers for Disease Control (CDC) Decrease in AIDS-related mortality in a state correctional system—New York, 1995–1998. MMWR Morb Mortal Wkly Rep. 1999;47(51–52):1115–1117. - PubMed
-
- Levasseur L, Marzo J, Ross N, Blatier C. Frequency of re-incarcerations in the same detention center: role of substitution therapy. A preliminary retrospective analysis. Ann Med Interne. 2002;153(3 Suppl):1S14–1S19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous