Induction of CD44 cleavage in articular chondrocytes
- PMID: 20178130
- PMCID: PMC2896278
- DOI: 10.1002/art.27410
Induction of CD44 cleavage in articular chondrocytes
Abstract
Objective: The hyaluronan receptor CD44 provides chondrocytes with a mechanism for sensing and responding to changes in the extracellular matrix. The purpose of this study was to document the fragmentation and loss of CD44 and to determine the likely mechanisms involved.
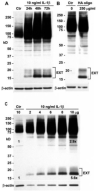
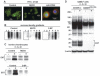
Methods: A polyclonal anti-CD44 cytotail antibody was generated to detect CD44 fragmentation by Western blot analysis. Chondrocytes were isolated from human or bovine articular cartilage. Primary articular chondrocytes were treated with interleukin-1beta (IL-1beta), hyaluronan oligosaccharides, or phorbol myristate acetate or were passaged and subcultured in monolayer to induce dedifferentiation. Conditions that altered the capacity of CD44 to transit into lipid rafts, or pharmacologic inhibitors of metalloproteinase or gamma-secretase activity were used to define the mechanism of fragmentation of CD44.
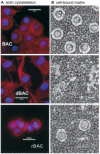
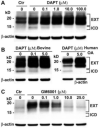
Results: Chondrocytes from osteoarthritic cartilage exhibited CD44 fragmentation as low molecular mass bands, corresponding to the CD44-EXT and CD44-ICD bands. Following dedifferentiation of chondrocytes or treatment of primary chondrocytes with hyaluronan oligosaccharides, IL-1beta, or phorbol myristate acetate, CD44 fragmentation was enhanced. Subsequent culture of the dedifferentiated chondrocytes in 3-dimensional alginate beads rescued the chondrocyte phenotype and diminished the fragmentation of CD44. Fragmentation of CD44 in chondrocytes was blocked in the presence of the metalloproteinase inhibitor GM6001 and the gamma-secretase inhibitor DAPT.
Conclusion: CD44 fragmentation, consistent with a signature pattern reported for sequential metalloproteinase/gamma-secretase cleavage of CD44, is a common metabolic feature of chondrocytes that have undergone dedifferentiation in vitro and osteoarthritic chondrocytes. Transit of CD44 into lipid rafts may be required for its fragmentation.
Figures


References
-
- Hardingham TE, Fosang AJ. Proteoglycans: many forms and many functions. FASEB J. 1992;6:861–70. - PubMed
-
- Knudson W, Knudson C. An update on hyaluronan and CD44 in cartilage. Current Opin Orthop. 2004;15:369–75.
-
- Nishida Y, Knudson CB, Nietfeld JJ, Margulis A, Knudson W. Antisense inhibition of hyaluronan synthase-2 in human articular chondrocytes inhibits proteoglycan retention and matrix assembly. J Biol Chem. 1999;274:21893–9. - PubMed
-
- Knudson CB, Knudson W. Hyaluronan-binding proteins in development, tissue homeostasis and disease. FASEB J. 1993;7:1233–41. - PubMed
-
- Thorne RF, Legg JW, Isacke CM. The role of the CD44 transmembrane and cytoplasmic domains in co-ordinating adhesive and signalling events. J Cell Sci. 2004;117:373–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

