Hypoxia-induced endothelial secretion of macrophage migration inhibitory factor and role in endothelial progenitor cell recruitment
- PMID: 20178462
- PMCID: PMC3922388
- DOI: 10.1111/j.1582-4934.2010.01041.x
Hypoxia-induced endothelial secretion of macrophage migration inhibitory factor and role in endothelial progenitor cell recruitment
Abstract
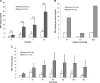
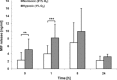
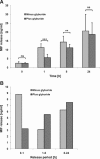
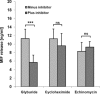
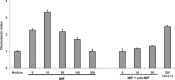
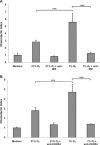
Macrophage migration inhibitory factor (MIF) is a pleiotropic inflammatory cytokine that was recently identified as a non-cognate ligand of the CXC-family chemokine receptors 2 and 4 (CXCR2 and CXCR4). MIF is expressed and secreted from endothelial cells (ECs) following atherogenic stimulation, exhibits chemokine-like properties and promotes the recruitment of leucocytes to atherogenic endothelium. CXCR4 expressed on endothelial progenitor cells (EPCs) and EC-derived CXCL12, the cognate ligand of CXCR4, have been demonstrated to be critical when EPCs are recruited to ischemic tissues. Here we studied whether hypoxic stimulation triggers MIF secretion from ECs and whether the MIF/CXCR4 axis contributes to EPC recruitment. Exposure of human umbilical vein endothelial cells (HUVECs) and human aortic endothelial cells (HAoECs) to 1% hypoxia led to the specific release of substantial amounts of MIF. Hypoxia-induced MIF release followed a biphasic behaviour. MIF secretion in the first phase peaked at 60 min. and was inhibited by glyburide, indicating that this MIF pool was secreted by a non-classical mechanism and originated from pre-formed MIF stores. Early hypoxia-triggered MIF secretion was not inhibited by cycloheximide and echinomycin, inhibitors of general and hypoxia-inducible factor (HIF)-1α-induced protein synthesis, respectively. A second phase of MIF secretion peaked around 8 hrs and was likely due to HIF-1α-induced de novo synthesis of MIF. To functionally investigate the role of hypoxia-inducible secreted MIF on the recruitment of EPCs, we subjected human AcLDL(+) KDR(+) CD31(+) EPCs to a chemotactic MIF gradient. MIF potently promoted EPC chemotaxis in a dose-dependent bell-shaped manner (peak: 10 ng/ml MIF). Importantly, EPC migration was induced by supernatants of hypoxia-conditioned HUVECs, an effect that was completely abrogated by anti-MIF- or anti-CXCR4-antibodies. Thus, hypoxia-induced MIF secretion from ECs might play an important role in the recruitment and migration of EPCs to hypoxic tissues such as after ischemia-induced myocardial damage.
© 2011 The Authors Journal of Cellular and Molecular Medicine © 2011 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures

References
-
- Bernhagen J, Calandra T, Bucala R. Regulation of the immune response by macrophage migration inhibitory factor: biological and structural features. J Mol Med. 1998;76:151–61. - PubMed
-
- Morand EF, Leech M, Bernhagen J. MIF: a new cytokine link between rheumatoid arthritis and atherosclerosis. Nat Rev Drug Discov. 2006;5:399–410. - PubMed
-
- Zernecke A, Bernhagen J, Weber C. Macrophage migration inhibitory factor in cardiovascular disease. Circulation. 2008;117:1594–602. - PubMed
-
- Bernhagen J, Krohn R, Lue H, et al. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat Med. 2007;13:587–96. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

