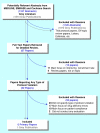
A systematic review of techniques and interventions for improving adherence to inclusion and exclusion criteria during enrolment into randomised controlled trials
- PMID: 20178571
- PMCID: PMC2838880
- DOI: 10.1186/1745-6215-11-17
A systematic review of techniques and interventions for improving adherence to inclusion and exclusion criteria during enrolment into randomised controlled trials
Abstract
Background: Enrolment of patients into a randomised controlled trial (RCT) in violation of key inclusion or exclusion criteria, may lead to excess avoidable harm. The purpose of this paper was to systematically identify and review techniques and interventions proven to prevent or avoid inappropriate enrolment of patients into RCTs.
Methods: EMBASE, MEDLINE, Cochrane Database of Systematic Reviews, Cochrane Methodology Register, online abstract repositories, and conference websites were searched. Experts were contacted and bibliographies of retrieved papers hand-searched. The search cut-off date was 31 August 2009.
Results: No primary publications were found. We identified one study in the grey literature (conference abstracts and presentations) reporting the results of an evaluation of the effectiveness of an intervention designed to prevent or avoid inappropriate enrolment of patients into an RCT. In the context of a multicentre trial, use of a dummy enrolment run-in phase was shown to reduce enrolment errors significantly (P < 0.001), from 16.1% during the run-in phase to < 1% after trial initiation.
Conclusions: Our systematic search yielded only one technique or intervention shown to improve adherence to eligibility criteria during enrolment into RCTs. Given the potential harm involved in recruiting patients into a clinical trial in violation of key eligibility criteria, future research is needed to better inform those conducting clinical trials of how best to prevent enrolment errors.
Figures
Similar articles
-
Structured treatment interruptions (STI) in chronic unsuppressed HIV infection in adults.Cochrane Database Syst Rev. 2006 Jul 19;2006(3):CD006148. doi: 10.1002/14651858.CD006148. Cochrane Database Syst Rev. 2006. PMID: 16856117 Free PMC article.
-
Individual-level interventions to reduce personal exposure to outdoor air pollution and their effects on people with long-term respiratory conditions.Cochrane Database Syst Rev. 2021 Aug 9;8(8):CD013441. doi: 10.1002/14651858.CD013441.pub2. Cochrane Database Syst Rev. 2021. PMID: 34368949 Free PMC article.
-
Interventions for the prevention and management of oropharyngeal candidiasis associated with HIV infection in adults and children.Cochrane Database Syst Rev. 2010 Nov 10;2010(11):CD003940. doi: 10.1002/14651858.CD003940.pub3. Cochrane Database Syst Rev. 2010. PMID: 21069679 Free PMC article.
-
Interventions for promoting habitual exercise in people living with and beyond cancer.Cochrane Database Syst Rev. 2018 Sep 19;9(9):CD010192. doi: 10.1002/14651858.CD010192.pub3. Cochrane Database Syst Rev. 2018. PMID: 30229557 Free PMC article.
-
Treatments for seizures in catamenial (menstrual-related) epilepsy.Cochrane Database Syst Rev. 2021 Sep 16;9(9):CD013225. doi: 10.1002/14651858.CD013225.pub3. Cochrane Database Syst Rev. 2021. PMID: 34528245 Free PMC article.
Cited by
-
Eligibility determination for clinical trials: development of a case review process at a chiropractic research center.Trials. 2014 Oct 24;15:406. doi: 10.1186/1745-6215-15-406. Trials. 2014. PMID: 25344427 Free PMC article.
-
Defining and Identifying Members of a Research Study Population: CTSA-Affiliated Faculty Members.Hypothesis (Macon). 2014 Spring;26(1):5-11. Hypothesis (Macon). 2014. PMID: 25067900 Free PMC article. No abstract available.
-
Evaluating the feasibility of a multicenter teleneonatology clinical effectiveness trial.Pediatr Res. 2023 Oct;94(4):1555-1561. doi: 10.1038/s41390-023-02659-2. Epub 2023 May 19. Pediatr Res. 2023. PMID: 37208433
-
SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials.BMJ. 2013 Jan 8;346:e7586. doi: 10.1136/bmj.e7586. BMJ. 2013. PMID: 23303884 Free PMC article.
-
[Enteral nutrition therapy in critical care : Current knowledge, controversies, and practical implementation].Med Klin Intensivmed Notfmed. 2016 May;111(4):330-40. doi: 10.1007/s00063-015-0048-5. Epub 2015 Jun 20. Med Klin Intensivmed Notfmed. 2016. PMID: 26091922 Review. German.
References
-
- Pocock SJ. Clinical Trials. A Practical Approach. Chichester, England: John Wiley and Sons Ltd; 1983.
-
- Macias WL, Vallet B, Bernard GR, Vincent JL, Laterre PF, Nelson DR, Nelson DR, Derchak PA, Dhainaut JF. Sources of variability on the estimate of treatment effect in the PROWESS trial: implications for the design and conduct of future studies in severe sepsis. Crit Care Med. 2004;32:2385–2391. doi: 10.1097/01.CCM.0000147440.71142.AC. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases