G-CSF protects motoneurons against axotomy-induced apoptotic death in neonatal mice
- PMID: 20178614
- PMCID: PMC2844381
- DOI: 10.1186/1471-2202-11-25
G-CSF protects motoneurons against axotomy-induced apoptotic death in neonatal mice
Abstract
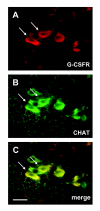
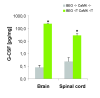
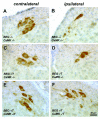
Background: Granulocyte colony stimulating factor (G-CSF) is a growth factor essential for generation of neutrophilic granulocytes. Apart from this hematopoietic function, we have recently uncovered potent neuroprotective and regenerative properties of G-CSF in the central nervous system (CNS). The G-CSF receptor and G-CSF itself are expressed in alpha motoneurons, G-CSF protects motoneurons, and improves outcome in the SOD1(G93A) transgenic mouse model for amyotrophic lateral sclerosis (ALS). In vitro, G-CSF acts anti-apoptotically on motoneuronal cells. Due to the pleiotrophic effects of G-CSF and the complexity of the SOD1 transgenic ALS models it was however not possible to clearly distinguish between directly mediated anti-apoptotic and indirectly protective effects on motoneurons. Here we studied whether G-CSF is able to protect motoneurons from purely apoptotic cell death induced by a monocausal paradigm, neonatal sciatic nerve axotomy.
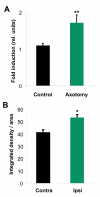
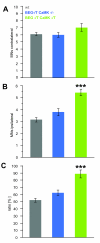
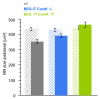
Results: We performed sciatic nerve axotomy in neonatal mice overexpressing G-CSF in the CNS and found that G-CSF transgenic mice displayed significantly higher numbers of surviving lumbar motoneurons 4 days following axotomy than their littermate controls. Also, surviving motoneurons in G-CSF overexpressing animals were larger, suggesting additional trophic effects of this growth factor.
Conclusions: In this model of pure apoptotic cell death the protective effects of G-CSF indicate direct actions of G-CSF on motoneurons in vivo. This shows that G-CSF exerts potent anti-apoptotic activities towards motoneurons in vivo and suggests that the protection offered by G-CSF in ALS mouse models is due to its direct neuroprotective activity.
Figures
Similar articles
-
Granulocyte-colony stimulating factor improves outcome in a mouse model of amyotrophic lateral sclerosis.Brain. 2008 Dec;131(Pt 12):3335-47. doi: 10.1093/brain/awn243. Epub 2008 Oct 3. Brain. 2008. PMID: 18835867 Free PMC article.
-
Neuroprotective effects of NGF, BDNF, NT-3 and GDNF on axotomized extraocular motoneurons in neonatal rats.Neuroscience. 2013 Oct 10;250:31-48. doi: 10.1016/j.neuroscience.2013.06.050. Epub 2013 Jul 2. Neuroscience. 2013. PMID: 23827308
-
CNS-targeted viral delivery of G-CSF in an animal model for ALS: improved efficacy and preservation of the neuromuscular unit.Mol Ther. 2011 Feb;19(2):284-92. doi: 10.1038/mt.2010.271. Epub 2010 Dec 7. Mol Ther. 2011. PMID: 21139572 Free PMC article.
-
Granulocyte-colony stimulating factor (G-CSF): an emerging therapeutic approach for amyotrophic lateral sclerosis (ALS).Acta Neurol Belg. 2023 Jun;123(3):763-771. doi: 10.1007/s13760-022-01996-z. Epub 2022 Jun 23. Acta Neurol Belg. 2023. PMID: 35737276 Review.
-
Neuroprotection through G-CSF: recent advances and future viewpoints.Pharmacol Rep. 2021 Apr;73(2):372-385. doi: 10.1007/s43440-020-00201-3. Epub 2021 Jan 2. Pharmacol Rep. 2021. PMID: 33389706 Review.
Cited by
-
Biomarker Supervised G-CSF (Filgrastim) Response in ALS Patients.Front Neurol. 2018 Nov 26;9:971. doi: 10.3389/fneur.2018.00971. eCollection 2018. Front Neurol. 2018. PMID: 30534107 Free PMC article.
-
G-CSF promotes autophagy and reduces neural tissue damage after spinal cord injury in mice.Lab Invest. 2015 Dec;95(12):1439-49. doi: 10.1038/labinvest.2015.120. Epub 2015 Nov 2. Lab Invest. 2015. PMID: 26524416
-
Zinc Improves Functional Recovery by Regulating the Secretion of Granulocyte Colony Stimulating Factor From Microglia/Macrophages After Spinal Cord Injury.Front Mol Neurosci. 2019 Feb 1;12:18. doi: 10.3389/fnmol.2019.00018. eCollection 2019. Front Mol Neurosci. 2019. PMID: 30774583 Free PMC article.
-
Granulocyte colony stimulating factor attenuates inflammation in a mouse model of amyotrophic lateral sclerosis.J Neuroinflammation. 2011 Jun 28;8:74. doi: 10.1186/1742-2094-8-74. J Neuroinflammation. 2011. PMID: 21711557 Free PMC article.
-
Cellular players of hematopoietic stem cell mobilization in the bone marrow niche.Int J Hematol. 2017 Feb;105(2):129-140. doi: 10.1007/s12185-016-2162-4. Epub 2016 Dec 10. Int J Hematol. 2017. PMID: 27943116 Review.
References
-
- Welte K, Platzer E, Gabrilove JL, Lu L, Levi E, Polivka A, Mertelsmann R, Moore MA. Purification to apparent homogeneity and biochemical characterization of human pluripotent hematopoietic colony-stimulating factor. Haematol Blood Transfus. 1985;29:398–401. - PubMed
-
- Kawada H, Takizawa S, Takanashi T, Morita Y, Fujita J, Fukuda K, Takagi S, Okano H, Ando K, Hotta T. Administration of hematopoietic cytokines in the subacute phase after cerebral infarction is effective for functional recovery facilitating proliferation of intrinsic neural stem/progenitor cells and transition of bone marrow-derived neuronal cells. Circulation. 2006;113(5):701–710. doi: 10.1161/CIRCULATIONAHA.105.563668. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

