The human PAF1 complex acts in chromatin transcription elongation both independently and cooperatively with SII/TFIIS
- PMID: 20178742
- PMCID: PMC2853908
- DOI: 10.1016/j.cell.2009.12.050
The human PAF1 complex acts in chromatin transcription elongation both independently and cooperatively with SII/TFIIS
Abstract
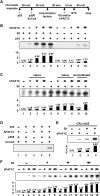
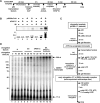
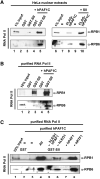
Genetic and cell-based studies have implicated the PAF1 complex (PAF1C) in transcription-associated events, but there has been no evidence showing a direct role in facilitating transcription of a natural chromatin template. Here, we demonstrate an intrinsic ability of human PAF1C (hPAF1C) to facilitate activator (p53)- and histone acetyltransferase (p300)-dependent transcription elongation from a recombinant chromatin template in a biochemically defined RNA polymerase II transcription system. This represents a PAF1C function distinct from its established role in histone ubiquitylation and methylation. Importantly, we further demonstrate a strong synergy between hPAF1C and elongation factor SII/TFIIS and an underlying mechanism involving direct hPAF1C-SII interactions and cooperative binding to RNA polymerase II. Apart from a distinct PAF1C function, the present observations provide a molecular mechanism for the cooperative function of distinct transcription elongation factors in chromatin transcription.
2010 Elsevier Inc. All rights reserved.
Figures




References
-
- Arndt KM, Kane CM. Running with RNA polymerase: eukaryotic transcript elongation. Trends Genet. 2003;19:543–550. - PubMed
-
- Betz JL, Chang M, Washburn TM, Porter SE, Mueller CL, Jaehning JA. Phenotypic analysis of Paf1/Pol II complex mutations reveals connections to cell cycle regulation, protein synthesis, and lipid and nucleic acid metabolism. Mol. Genet. Genomics. 2002;268:272–285. - PubMed
-
- Bondarenko VA, Steele LM, Ujvari A, Gaykalova DA, Kulaeva OI, Polikanov YS, Luse DS, Studitsky VM. Nucleosomes can form a polar barrier to transcript elongation by Pol II. Mol. Cell. 2006;24:469–479. - PubMed
-
- Chaudhary K, Deb S, Moniaux N, Ponnusamy MP, Batra SK. Human RNA polymerase II-associated factor complex: dysregulation in cancer. Oncogene. 2007;26:7499–7507. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

