Interaction of Vpx and apolipoprotein B mRNA-editing catalytic polypeptide 3 family member A (APOBEC3A) correlates with efficient lentivirus infection of monocytes
- PMID: 20178977
- PMCID: PMC2852964
- DOI: 10.1074/jbc.M109.090977
Interaction of Vpx and apolipoprotein B mRNA-editing catalytic polypeptide 3 family member A (APOBEC3A) correlates with efficient lentivirus infection of monocytes
Abstract
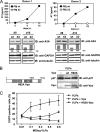
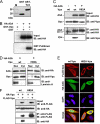
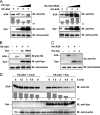
The accessory protein Vpx is encoded by lentiviruses of the human immunodeficiency virus type 2 (HIV-2) and the simian immunodeficiency SIVsm/SIVmac lineage. It is packaged into virions and is indispensable in early steps of monocyte infection. HIV-1, which does not encode Vpx, is not able to infect human monocytes, but Vpx enables infection with HIV-1. The underlying mechanism is not completely understood. In this work, we focus on Vpx-mediated intracellular postentry events as counteraction of host cell proteins. We found that Vpx binds to apolipoprotein B mRNA-editing catalytic polypeptide 3 family member A (APOBEC3A; A3A), a member of the family of cytidine deaminases, present in monocytes. This interaction led to a reduction of the steady-state protein level of A3A. A single-point mutation in Vpx (H82A) abrogated binding to A3A and single-round infection of monocytes by HIV-1. Taken together, our data indicate that lentiviral Vpx counteracts A3A in human monocytes.
Figures
Similar articles
-
Human immunodeficiency virus type 1 modified to package Simian immunodeficiency virus Vpx efficiently infects macrophages and dendritic cells.J Virol. 2011 Jul;85(13):6263-74. doi: 10.1128/JVI.00346-11. Epub 2011 Apr 20. J Virol. 2011. PMID: 21507971 Free PMC article.
-
Restriction of HIV-1 replication in monocytes is abolished by Vpx of SIVsmmPBj.PLoS One. 2009 Sep 21;4(9):e7098. doi: 10.1371/journal.pone.0007098. PLoS One. 2009. PMID: 19768115 Free PMC article.
-
Vpx overcomes a SAMHD1-independent block to HIV reverse transcription that is specific to resting CD4 T cells.Proc Natl Acad Sci U S A. 2017 Mar 7;114(10):2729-2734. doi: 10.1073/pnas.1613635114. Epub 2017 Feb 22. Proc Natl Acad Sci U S A. 2017. PMID: 28228523 Free PMC article.
-
Limelight on two HIV/SIV accessory proteins in macrophage infection: is Vpx overshadowing Vpr?Retrovirology. 2010 Apr 9;7:35. doi: 10.1186/1742-4690-7-35. Retrovirology. 2010. PMID: 20380700 Free PMC article. Review.
-
[Research Progress in Viral Protein Vpx induction of Proteasomal Degradation of the Antiviral Factor SAMHD1].Bing Du Xue Bao. 2016 May;32(3):355-60. Bing Du Xue Bao. 2016. PMID: 29963825 Review. Chinese.
Cited by
-
An ancient history of gene duplications, fusions and losses in the evolution of APOBEC3 mutators in mammals.BMC Evol Biol. 2012 May 28;12:71. doi: 10.1186/1471-2148-12-71. BMC Evol Biol. 2012. PMID: 22640020 Free PMC article.
-
TLR7/8 agonist induces a post-entry SAMHD1-independent block to HIV-1 infection of monocytes.Retrovirology. 2016 Dec 1;13(1):83. doi: 10.1186/s12977-016-0316-3. Retrovirology. 2016. PMID: 27905985 Free PMC article.
-
APOBEC3A is a specific inhibitor of the early phases of HIV-1 infection in myeloid cells.PLoS Pathog. 2011 Sep;7(9):e1002221. doi: 10.1371/journal.ppat.1002221. Epub 2011 Sep 22. PLoS Pathog. 2011. PMID: 21966267 Free PMC article.
-
A DNA sequence recognition loop on APOBEC3A controls substrate specificity.PLoS One. 2014 May 14;9(5):e97062. doi: 10.1371/journal.pone.0097062. eCollection 2014. PLoS One. 2014. PMID: 24827831 Free PMC article.
-
Vpx rescues HIV-1 transduction of dendritic cells from the antiviral state established by type 1 interferon.Retrovirology. 2011 Jun 22;8:49. doi: 10.1186/1742-4690-8-49. Retrovirology. 2011. PMID: 21696578 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

