cAMP/CREB-mediated transcriptional regulation of ectonucleoside triphosphate diphosphohydrolase 1 (CD39) expression
- PMID: 20178980
- PMCID: PMC2863166
- DOI: 10.1074/jbc.M110.116905
cAMP/CREB-mediated transcriptional regulation of ectonucleoside triphosphate diphosphohydrolase 1 (CD39) expression
Abstract
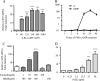
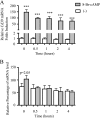
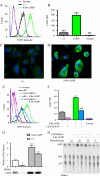
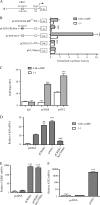
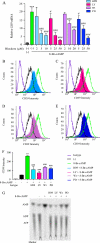
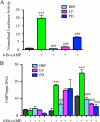
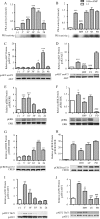
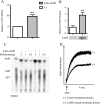
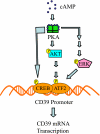
CD39 is a transmembrane enzyme that inhibits platelet reactivity and inflammation by phosphohydrolyzing ATP and ADP to AMP. Cyclic AMP (cAMP), an essential second messenger, is particularly important in regulating genes controlling vascular homeostasis. These experiments test the hypothesis that cAMP might positively regulate the expression of CD39 and thereby modulate important vascular homeostatic properties. Cd39 mRNA was induced by 13.8- fold in RAW cells treated with a membrane-permeant cAMP analogue (8-bromo-cyclic AMP; 8-Br-cAMP), stimulation of adenylate cyclase, or prostanoids known to drive cAMP response. Fluorescence-activated cell sorting, immunofluorescence, and TLC assays demonstrated that both CD39 protein expression and enzymatic activity were increased in cells treated with 8-Br-cAMP but not in cells transfected with short hairpin RNA against CD39. This analogue drove a significant increase in transcriptional activity at the Cd39 promoter although not when the promoter's cAMP-response element sites were mutated. Pretreatment with cAMP-dependent protein kinase (PKA), phosphoinositide 3-kinase (PI3K), or ERK inhibitors nearly obliterated the cAMP-driven increase in Cd39 mRNA, protein expression, and promoter activity. 8-Br-cAMP greatly increased the phosphorylation of CREB1 (Ser(133)) and ATF2 (Thr(71)) in a PKA-, PI3K-, and ERK-dependent fashion. Chromatin immunoprecipitation assays demonstrated that binding of phosphorylated CREB1 and ATF2 to cAMP-response element-like sites was significantly increased with 8-Br-cAMP treatment and that binding was reduced with PKA, PI3K, and ERK inhibition, whereas transfection of Creb1 and Atf2 overexpression constructs enhanced cAMP-driven Cd39 mRNA expression. Transfection of RAW cells with mutated Creb1 (S133A) reduced cAMP-driven Cd39 mRNA expression. Furthermore, the cAMP-mediated induction of Cd39 mRNA, protein, and phosphohydrolytic activity was replicated in primary peritoneal macrophages. These data identify cAMP as a crucial regulator of macrophage CD39 expression and demonstrate that cAMP acts through the PKA/CREB, PKA/PI3K/ATF2, and PKA/ERK/ATF2 pathways to control a key vascular homeostatic mediator.
Figures
Similar articles
-
Mdivi-1 Protects Against Ischemic Brain Injury via Elevating Extracellular Adenosine in a cAMP/CREB-CD39-Dependent Manner.Mol Neurobiol. 2016 Jan;53(1):240-253. doi: 10.1007/s12035-014-9002-4. Epub 2014 Nov 27. Mol Neurobiol. 2016. PMID: 25428621
-
CREB/PKA sensitive signalling pathways activate and maintain expression levels of the hepatitis B virus pre-S2/S promoter.Gut. 2005 Sep;54(9):1309-17. doi: 10.1136/gut.2005.065086. Epub 2005 May 4. Gut. 2005. PMID: 15871998 Free PMC article.
-
CREB is required for cAMP/PKA signals upregulating neuropathy target esterase expression.DNA Cell Biol. 2013 Apr;32(4):199-205. doi: 10.1089/dna.2012.1835. Epub 2013 Mar 21. DNA Cell Biol. 2013. PMID: 23517531 Free PMC article.
-
Metabolic control of excessive extracellular nucleotide accumulation by CD39/ecto-nucleotidase-1: implications for ischemic vascular diseases.J Pharmacol Exp Ther. 2003 Apr;305(1):9-16. doi: 10.1124/jpet.102.043729. J Pharmacol Exp Ther. 2003. PMID: 12649347 Review.
-
The molecular biology of memory: cAMP, PKA, CRE, CREB-1, CREB-2, and CPEB.Mol Brain. 2012 May 14;5:14. doi: 10.1186/1756-6606-5-14. Mol Brain. 2012. PMID: 22583753 Free PMC article. Review.
Cited by
-
Conversion of extracellular ATP into adenosine: a master switch in renal health and disease.Nat Rev Nephrol. 2020 Sep;16(9):509-524. doi: 10.1038/s41581-020-0304-7. Epub 2020 Jul 8. Nat Rev Nephrol. 2020. PMID: 32641760 Review.
-
Extracellular adenosine generation in the regulation of pro-inflammatory responses and pathogen colonization.Biomolecules. 2015 May 5;5(2):775-92. doi: 10.3390/biom5020775. Biomolecules. 2015. PMID: 25950510 Free PMC article. Review.
-
Targeting Adenosine in Cancer Immunotherapy to Enhance T-Cell Function.Front Immunol. 2019 Jun 6;10:925. doi: 10.3389/fimmu.2019.00925. eCollection 2019. Front Immunol. 2019. PMID: 31244820 Free PMC article. Review.
-
Human macrophage SCN5A activates an innate immune signaling pathway for antiviral host defense.J Biol Chem. 2014 Dec 19;289(51):35326-40. doi: 10.1074/jbc.M114.611962. Epub 2014 Nov 3. J Biol Chem. 2014. PMID: 25368329 Free PMC article.
-
Purinergic Signaling to Terminate TLR Responses in Macrophages.Front Immunol. 2016 Mar 2;7:74. doi: 10.3389/fimmu.2016.00074. eCollection 2016. Front Immunol. 2016. PMID: 26973651 Free PMC article. Review.
References
-
- Marcus A. J., Broekman M. J., Drosopoulos J. H., Olson K. E., Islam N., Pinsky D. J., Levi R. (2005) Semin. Thromb. Hemost. 31, 234–246 - PubMed
-
- Marcus A. J., Broekman M. J., Drosopoulos J. H., Islam N., Pinsky D. J., Sesti C., Levi R. (2003) J. Pharmacol. Exp. Ther. 305, 9–16 - PubMed
-
- Marcus A. J., Broekman M. J., Drosopoulos J. H., Islam N., Pinsky D. J., Sesti C., Levi R. (2003) J. Thromb. Haemost. 1, 2497–2509 - PubMed
-
- Atkinson B., Dwyer K., Enjyoji K., Robson S. C. (2006) Blood Cells Mol. Dis. 36, 217–222 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

