Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and Tau: effects on cognitive impairments
- PMID: 20178983
- PMCID: PMC2857107
- DOI: 10.1074/jbc.M110.100420
Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and Tau: effects on cognitive impairments
Abstract
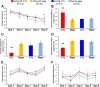
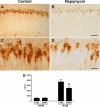
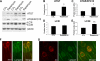
Accumulation of amyloid-beta (Abeta) and Tau is an invariant feature of Alzheimer disease (AD). The upstream role of Abeta accumulation in the disease pathogenesis is widely accepted, and there is strong evidence showing that Abeta accumulation causes cognitive impairments. However, the molecular mechanisms linking Abeta to cognitive decline remain to be elucidated. Here we show that the buildup of Abeta increases the mammalian target of rapamycin (mTOR) signaling, whereas decreasing mTOR signaling reduces Abeta levels, thereby highlighting an interrelation between mTOR signaling and Abeta. The mTOR pathway plays a central role in controlling protein homeostasis and hence, neuronal functions; indeed mTOR signaling regulates different forms of learning and memory. Using an animal model of AD, we show that pharmacologically restoring mTOR signaling with rapamycin rescues cognitive deficits and ameliorates Abeta and Tau pathology by increasing autophagy. Indeed, we further show that autophagy induction is necessary for the rapamycin-mediated reduction in Abeta levels. The results presented here provide a molecular basis for the Abeta-induced cognitive deficits and, moreover, show that rapamycin, an FDA approved drug, improves learning and memory and reduces Abeta and Tau pathology.
Figures

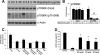

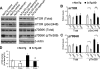

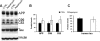
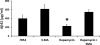
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

