Fas apoptosis inhibitory molecule regulates T cell receptor-mediated apoptosis of thymocytes by modulating Akt activation and Nur77 expression
- PMID: 20178987
- PMCID: PMC2852919
- DOI: 10.1074/jbc.M109.072744
Fas apoptosis inhibitory molecule regulates T cell receptor-mediated apoptosis of thymocytes by modulating Akt activation and Nur77 expression
Abstract
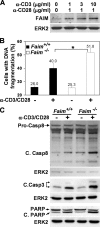
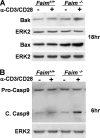
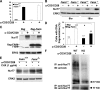
Fas apoptosis inhibitory molecule (FAIM) has been demonstrated to confer resistance to Fas-induced apoptosis of lymphocytes and hepatocytes in vitro and in vivo. Here, we show that FAIM is up-regulated in thymocytes upon T cell receptor (TCR) engagement and that faim(-/-) thymocytes are highly susceptible to TCR-mediated apoptosis with increased activation of caspase-8 and -9. Furthermore, injection of anti-CD3 antibodies leads to augmented depletion of CD4(+)CD8(+) T cells in the thymus of faim(-/-) mice compared with wild-type control, suggesting that FAIM plays a role in thymocyte apoptosis. Cross-linking of the TCR on faim(-/-) thymocytes leads to an elevated protein level of the orphan nuclear receptor Nur77, which plays a role in thymocyte apoptosis. Interestingly, in the absence of FAIM, there are reduced ubiquitination and degradation of the Nur77 protein. Faim(-/-) thymocytes also exhibit a defective TCR-induced activation of Akt whose activity we now show is required for Nur77 ubiquitination. Further analyses utilizing FAIM-deficient primary thymocytes and FAIM-overexpressing DO-11.10 T cells indicate that FAIM acts upstream of Akt during TCR signaling and influences the localization of Akt to lipid rafts, hence affecting its activation. Taken together, our study defined a TCR-induced FAIM/Akt/Nur77 signaling axis that is critical for modulating the apoptosis of developing thymocytes.
Figures



Similar articles
-
Differential roles for Bim and Nur77 in thymocyte clonal deletion induced by ubiquitous self-antigen.J Immunol. 2015 Mar 15;194(6):2643-53. doi: 10.4049/jimmunol.1400030. Epub 2015 Feb 16. J Immunol. 2015. PMID: 25687757
-
Immature CD4+CD8+ thymocytes and mature T cells regulate Nur77 distinctly in response to TCR stimulation.J Immunol. 2006 Nov 15;177(10):6660-6. doi: 10.4049/jimmunol.177.10.6660. J Immunol. 2006. PMID: 17082578
-
Genetic deletion of faim reveals its role in modulating c-FLIP expression during CD95-mediated apoptosis of lymphocytes and hepatocytes.Cell Death Differ. 2009 Jul;16(7):1062-70. doi: 10.1038/cdd.2009.26. Epub 2009 Mar 20. Cell Death Differ. 2009. PMID: 19300454
-
FAIM: An Antagonist of Fas-Killing and Beyond.Cells. 2019 Jun 4;8(6):541. doi: 10.3390/cells8060541. Cells. 2019. PMID: 31167518 Free PMC article. Review.
-
[The roles of orphan nuclear receptors in T-lymphocyte development in the thymus].Postepy Hig Med Dosw (Online). 2009 Oct 29;63:522-36. Postepy Hig Med Dosw (Online). 2009. PMID: 19940330 Review. Polish.
Cited by
-
FAIM regulates autophagy through glutaminolysis in lung adenocarcinoma.Autophagy. 2022 Jun;18(6):1416-1432. doi: 10.1080/15548627.2021.1987672. Epub 2021 Oct 31. Autophagy. 2022. PMID: 34720024 Free PMC article.
-
ASK1 Mediates Nur77 Expression in T-Cell Receptor Mediated Thymocyte Apoptosis.Cells. 2020 Mar 1;9(3):585. doi: 10.3390/cells9030585. Cells. 2020. PMID: 32121597 Free PMC article.
-
Sesamin Induces MCL-1-Dependent Apoptosis in Activated T Cells and Ameliorates Experimental Atopic Dermatitis.Int J Biol Sci. 2025 Jul 24;21(11):4719-4735. doi: 10.7150/ijbs.116753. eCollection 2025. Int J Biol Sci. 2025. PMID: 40860193 Free PMC article.
-
Apoptosis of pro-B lymphocytes induced by NR4A1 activation in the presence of gingival fibroblast exosomes and TNFα, caspase 8, STAT3, and Akt pathways modulators.Rom J Morphol Embryol. 2023 Jan-Mar;64(1):35-40. doi: 10.47162/RJME.64.1.04. Rom J Morphol Embryol. 2023. PMID: 37128789 Free PMC article.
-
Zebrafish screen identifies novel compound with selective toxicity against leukemia.Blood. 2012 Jun 14;119(24):5621-31. doi: 10.1182/blood-2011-12-398818. Epub 2012 Apr 9. Blood. 2012. PMID: 22490804 Free PMC article.
References
-
- Rathmell J. C., Thompson C. B. (2002) Cell 109, S97–107 - PubMed
-
- Sohn S. J., Rajpal A., Winoto A. (2003) Curr. Opin. Immunol. 15, 209–216 - PubMed
-
- Dhein J., Walczak H., Bäumler C., Debatin K. M., Krammer P. H. (1995) Nature 373, 438–441 - PubMed
-
- Moulian N., Berrih-Aknin S. (1998) Semin. Immunol. 10, 449–456 - PubMed
-
- Werlen G., Hausmann B., Naeher D., Palmer E. (2003) Science 299, 1859–1863 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

