CD271 antigen defines a subset of multipotent stromal cells with immunosuppressive and lymphohematopoietic engraftment-promoting properties
- PMID: 20179086
- PMCID: PMC2857196
- DOI: 10.3324/haematol.2009.015065
CD271 antigen defines a subset of multipotent stromal cells with immunosuppressive and lymphohematopoietic engraftment-promoting properties
Abstract
Background In vitro proliferative and differentiation potential of mesenchymal stromal cells generated from CD271(+) bone marrow mononuclear cells (CD271-mesenchymal stromal cells) has been demonstrated in several earlier and recent reports. In the present study we focused, in addition to proliferative and differentiation potential, on in vitro and in vivo immunosuppressive and lymphohematopoietic engraftment-promoting potential of these mesenchymal stromal cells compared to bone marrow-derived mesenchymal stromal cells generated by plastic adherence (plastic adherence-mesenchymal stromal cells).
Design and methods: We set up a series of experimental protocols in order to determine the phenotype of CD271-mesenchymal stromal cells, and their clonogenic, proliferative, differentiation and immunosuppressive potential. The potential of CD271-mesenchymal stromal cells to improve the engraftment of CD133(+) hematopoietic stem cells at co-transplantation was evaluated in immunodeficient NOD/SCID-IL2Rgamma(null) mice.
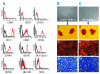
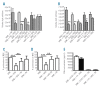
Results: In vitro studies demonstrated that CD271-mesenchymal stromal cells differentiate along adipogenic, osteogenic and chondrogenic lineages (trilineage potential), produce significantly higher levels of cytokines than plastic adherence-mesenchymal stromal cells, and significantly inhibit the proliferation of allogeneic T-lymphocytes in mixed lymphocyte reaction assays. Elevated levels of prostaglandin E(2), but not nitric monoxide, mediated the majority of this immunosuppressive effect. In vivo studies showed that CD271-mesenchymal stromal cells promoted significantly greater lymphoid engraftment than did plastic adherence-mesenchymal stromal cells when co-transplanted with CD133(+) hematopoietic stem cells at a ratio of 8:1 in immunodeficient NOD/SCID-IL2Rgamma(null) mice. They induced a 10.4-fold increase in the number of T cells, a 2.5-fold increase in the number of NK cells, and a 3.6-fold increase in the number of B cells, indicating a major qualitative difference between these two mesenchymal stromal cell populations. Conclusions Our results indicate that CD271 antigen provides a versatile marker for prospective isolation and expansion of multipotent mesenchymal stromal cells with immunosuppressive and lymphohematopoietic engraftment-promoting properties. The co-transplantation of such cells together with hematopoietic stem cells in patients with hematologic malignancies may prove valuable in the prevention of impaired/delayed T-cell recovery and graft-versus-host disease.
Figures


Similar articles
-
Clonal analysis of multipotent stromal cells derived from CD271+ bone marrow mononuclear cells: functional heterogeneity and different mechanisms of allosuppression.Haematologica. 2013 Oct;98(10):1609-16. doi: 10.3324/haematol.2013.092700. Epub 2013 Aug 23. Haematologica. 2013. PMID: 23975178 Free PMC article.
-
Selection of CD271(+) cells and human AB serum allows a large expansion of mesenchymal stromal cells from human bone marrow.Cytotherapy. 2009;11(2):153-62. doi: 10.1080/14653240802582125. Cytotherapy. 2009. PMID: 19301169
-
Potential Effect of CD271 on Human Mesenchymal Stromal Cell Proliferation and Differentiation.Int J Mol Sci. 2015 Jul 9;16(7):15609-24. doi: 10.3390/ijms160715609. Int J Mol Sci. 2015. PMID: 26184166 Free PMC article.
-
CD271 as a marker to identify mesenchymal stem cells from diverse sources before culture.World J Stem Cells. 2015 Mar 26;7(2):470-6. doi: 10.4252/wjsc.v7.i2.470. World J Stem Cells. 2015. PMID: 25815130 Free PMC article. Review.
-
Coaxing bone marrow stromal mesenchymal stem cells towards neuronal differentiation: progress and uncertainties.Cell Mol Life Sci. 2006 Jul;63(14):1649-57. doi: 10.1007/s00018-006-6019-5. Cell Mol Life Sci. 2006. PMID: 16786223 Free PMC article. Review.
Cited by
-
Bone Marrow-Derived Mesenchymal Stromal Cells: A Novel Target to Optimize Hematopoietic Stem Cell Transplantation Protocols in Hematological Malignancies and Rare Genetic Disorders.J Clin Med. 2019 Dec 18;9(1):2. doi: 10.3390/jcm9010002. J Clin Med. 2019. PMID: 31861268 Free PMC article. Review.
-
The Middle Part of the Plucked Hair Follicle Outer Root Sheath Is Identified as an Area Rich in Lineage-Specific Stem Cell Markers.Biomolecules. 2021 Jan 25;11(2):154. doi: 10.3390/biom11020154. Biomolecules. 2021. PMID: 33503918 Free PMC article.
-
CD271+ Mesenchymal Stem Cells as a Possible Infectious Niche for Leishmania infantum.PLoS One. 2016 Sep 13;11(9):e0162927. doi: 10.1371/journal.pone.0162927. eCollection 2016. PLoS One. 2016. PMID: 27622907 Free PMC article.
-
A novel antagonist of p75NTR reduces peripheral expansion and CNS trafficking of pro-inflammatory monocytes and spares function after traumatic brain injury.J Neuroinflammation. 2016 Apr 22;13(1):88. doi: 10.1186/s12974-016-0544-4. J Neuroinflammation. 2016. PMID: 27102880 Free PMC article.
-
Clonal analysis of multipotent stromal cells derived from CD271+ bone marrow mononuclear cells: functional heterogeneity and different mechanisms of allosuppression.Haematologica. 2013 Oct;98(10):1609-16. doi: 10.3324/haematol.2013.092700. Epub 2013 Aug 23. Haematologica. 2013. PMID: 23975178 Free PMC article.
References
-
- Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3(4):393–403. - PubMed
-
- Dicker A, Le Blanc K, Aström G, van Harmelen V, Götherström C, Blomqvist L, et al. Functional studies of mesenchymal stem cells derived from adult human adipose tissue. Exp Cell Res. 2005;308(2):283–90. - PubMed
-
- Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, Fisk NM. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98(8):2396–402. - PubMed
-
- in 't Anker PS, Noort WA, Scherjon SA, Kleijburg-van der Keur C, Kruisselbrink AB, van Bezooijen RL, et al. Mesenchymal stem cells in human second-trimester bone marrow, liver, lung, and spleen exhibit a similar immunophenotype but a heterogeneous multilineage differentiation potential. Haematologica. 2003;88(8):845–52. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

