Arabidopsis S-sulfocysteine synthase activity is essential for chloroplast function and long-day light-dependent redox control
- PMID: 20179139
- PMCID: PMC2845405
- DOI: 10.1105/tpc.109.071985
Arabidopsis S-sulfocysteine synthase activity is essential for chloroplast function and long-day light-dependent redox control
Abstract
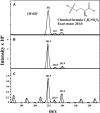
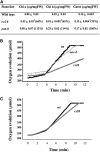
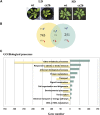
In bacteria, the biosynthesis of Cys is accomplished by two enzymes that are encoded by the cysK and cysM genes. CysM is also able to use thiosulfate as a substrate to produce S-sulfocysteine. In plant cells, the biosynthesis of Cys occurs in the cytosol, mitochondria, and chloroplasts. Chloroplasts contain two O-acetylserine(thiol)lyase homologs, which are encoded by the OAS-B and CS26 genes in Arabidopsis thaliana. An in vitro enzymatic analysis of the recombinant CS26 protein demonstrated that this isoform possesses S-sulfocysteine synthase activity and lacks O-acetylserine(thiol)lyase activity. In vivo functional analysis of this enzyme in knockout mutants demonstrated that mutation of CS26 suppressed the S-sulfocysteine synthase activity that was detected in the wild type; furthermore, the cs26 mutants exhibited a reduction in size and showed paleness, but penetrance of the growth phenotype depended on the light regime. The cs26 mutant plants also had reductions in chlorophyll content and photosynthetic activity (neither of which were observed in oas-b mutants) as well as elevated glutathione levels. However, cs26 leaves were not able to properly detoxify reactive oxygen species, which accumulated to high levels under long-day growth conditions. The transcriptional profile of the cs26 mutant revealed that the mutation had a pleiotropic effect on many cellular and metabolic processes. Our findings reveal that S-sulfocysteine and the activity of S-sulfocysteine synthase play important roles in chloroplast function and are essential for light-dependent redox regulation within the chloroplast.
Figures






Similar articles
-
Photosynthetic adaptation to length of day is dependent on S-sulfocysteine synthase activity in the thylakoid lumen.Plant Physiol. 2012 Sep;160(1):274-88. doi: 10.1104/pp.112.201491. Epub 2012 Jul 24. Plant Physiol. 2012. PMID: 22829322 Free PMC article.
-
S-sulfocysteine synthase function in sensing chloroplast redox status.Plant Signal Behav. 2013 Mar;8(3):e23313. doi: 10.4161/psb.23313. Epub 2013 Jan 18. Plant Signal Behav. 2013. PMID: 23333972 Free PMC article.
-
GRA78 encoding a putative S-sulfocysteine synthase is involved in chloroplast development at the early seedling stage of rice.Plant Sci. 2019 Mar;280:321-329. doi: 10.1016/j.plantsci.2018.12.019. Epub 2018 Dec 21. Plant Sci. 2019. PMID: 30824011
-
Cysteine and cysteine-related signaling pathways in Arabidopsis thaliana.Mol Plant. 2014 Feb;7(2):264-76. doi: 10.1093/mp/sst168. Epub 2013 Nov 27. Mol Plant. 2014. PMID: 24285094 Review.
-
Plant Chloroplast Stress Response: Insights from Thiol Redox Proteomics.Antioxid Redox Signal. 2020 Jul 1;33(1):35-57. doi: 10.1089/ars.2019.7823. Epub 2020 Mar 12. Antioxid Redox Signal. 2020. PMID: 31989831 Review.
Cited by
-
Transient transcriptional regulation of the CYS-C1 gene and cyanide accumulation upon pathogen infection in the plant immune response.Plant Physiol. 2013 Aug;162(4):2015-27. doi: 10.1104/pp.113.219436. Epub 2013 Jun 19. Plant Physiol. 2013. PMID: 23784464 Free PMC article.
-
Comparison of transcriptional changes to chloroplast and mitochondrial perturbations reveals common and specific responses in Arabidopsis.Front Plant Sci. 2012 Dec 24;3:281. doi: 10.3389/fpls.2012.00281. eCollection 2012. Front Plant Sci. 2012. PMID: 23269925 Free PMC article.
-
A spinach O-acetylserine(thiol)lyase homologue, SoCSaseLP, suppresses cysteine biosynthesis catalysed by other enzyme isoforms.Biochim Open. 2016 Feb 8;2:24-32. doi: 10.1016/j.biopen.2016.01.002. eCollection 2016 Jun. Biochim Open. 2016. PMID: 29632835 Free PMC article.
-
Stress-responsive pathways and small RNA changes distinguish variable developmental phenotypes caused by MSH1 loss.BMC Plant Biol. 2017 Feb 20;17(1):47. doi: 10.1186/s12870-017-0996-4. BMC Plant Biol. 2017. PMID: 28219335 Free PMC article.
-
Universal and taxon-specific trends in protein sequences as a function of age.Elife. 2021 Jan 8;10:e57347. doi: 10.7554/eLife.57347. Elife. 2021. PMID: 33416492 Free PMC article.
References
-
- Barroso C., Vega J.M., Gotor C. (1995). A new member of the cytosolic O-acetylserine(thiol)lyase gene family in Arabidopsis thaliana. FEBS Lett. 363: 1–5 - PubMed
-
- Benjamini Y., Hochberg Y. (1995). Controlling the false discovery rate - a practical and powerful approach to multiple testing. J. R. Stat. Soc., B 57: 289–300
-
- Bonner E.R., Cahoon R.E., Knapke S.M., Jez J.M. (2005). Molecular basis of cysteine biosynthesis in plants - Structural and functional analysis of O-acetylserine sulfhydrylase from Arabidopsis thaliana. J. Biol. Chem. 280: 38803–38813 - PubMed
-
- Bradford M.M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

