Arabidopsis S-sulfocysteine synthase activity is essential for chloroplast function and long-day light-dependent redox control
- PMID: 20179139
- PMCID: PMC2845405
- DOI: 10.1105/tpc.109.071985
Arabidopsis S-sulfocysteine synthase activity is essential for chloroplast function and long-day light-dependent redox control
Abstract
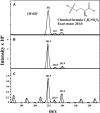
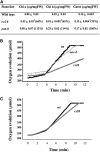
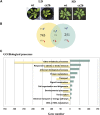
In bacteria, the biosynthesis of Cys is accomplished by two enzymes that are encoded by the cysK and cysM genes. CysM is also able to use thiosulfate as a substrate to produce S-sulfocysteine. In plant cells, the biosynthesis of Cys occurs in the cytosol, mitochondria, and chloroplasts. Chloroplasts contain two O-acetylserine(thiol)lyase homologs, which are encoded by the OAS-B and CS26 genes in Arabidopsis thaliana. An in vitro enzymatic analysis of the recombinant CS26 protein demonstrated that this isoform possesses S-sulfocysteine synthase activity and lacks O-acetylserine(thiol)lyase activity. In vivo functional analysis of this enzyme in knockout mutants demonstrated that mutation of CS26 suppressed the S-sulfocysteine synthase activity that was detected in the wild type; furthermore, the cs26 mutants exhibited a reduction in size and showed paleness, but penetrance of the growth phenotype depended on the light regime. The cs26 mutant plants also had reductions in chlorophyll content and photosynthetic activity (neither of which were observed in oas-b mutants) as well as elevated glutathione levels. However, cs26 leaves were not able to properly detoxify reactive oxygen species, which accumulated to high levels under long-day growth conditions. The transcriptional profile of the cs26 mutant revealed that the mutation had a pleiotropic effect on many cellular and metabolic processes. Our findings reveal that S-sulfocysteine and the activity of S-sulfocysteine synthase play important roles in chloroplast function and are essential for light-dependent redox regulation within the chloroplast.
Figures






References
-
- Barroso C., Vega J.M., Gotor C. (1995). A new member of the cytosolic O-acetylserine(thiol)lyase gene family in Arabidopsis thaliana. FEBS Lett. 363: 1–5 - PubMed
-
- Benjamini Y., Hochberg Y. (1995). Controlling the false discovery rate - a practical and powerful approach to multiple testing. J. R. Stat. Soc., B 57: 289–300
-
- Bonner E.R., Cahoon R.E., Knapke S.M., Jez J.M. (2005). Molecular basis of cysteine biosynthesis in plants - Structural and functional analysis of O-acetylserine sulfhydrylase from Arabidopsis thaliana. J. Biol. Chem. 280: 38803–38813 - PubMed
-
- Bradford M.M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

