CD73 on tumor cells impairs antitumor T-cell responses: a novel mechanism of tumor-induced immune suppression
- PMID: 20179192
- PMCID: PMC2883609
- DOI: 10.1158/0008-5472.CAN-09-3109
CD73 on tumor cells impairs antitumor T-cell responses: a novel mechanism of tumor-induced immune suppression
Abstract
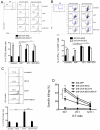
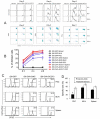
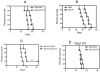
CD73, originally defined as a lymphocyte differentiation antigen, is thought to function as a cosignaling molecule on T lymphocytes and an adhesion molecule that is required for lymphocyte binding to endothelium. We show here that CD73 is widely expressed on many tumor cell lines and is upregulated in cancerous tissues. Because the ecto-5'-nucleotidase activity of CD73 catalyzes AMP breakdown to immunosuppressive adenosine, we hypothesized that CD73-generated adenosine prevents tumor destruction by inhibiting antitumor immunity. We confirmed this hypothesis by showing that combining tumor CD73 knockdown and tumor-specific T-cell transfer cured all tumor-bearing mice. In striking contrast, there was no therapeutic benefit of adoptive T-cell immunotherapy in mice bearing tumors without CD73 knockdown. Moreover, blockade of the A2A adenosine receptor with a selective antagonist also augmented the efficacy of adoptive T-cell therapy. These findings identify a potential mechanism for CD73-mediated tumor immune evasion and point to a novel cancer immunotherapy strategy by targeting the enzymatic activity of tumor CD73.
Figures



Similar articles
-
Blockade of A2A receptors potently suppresses the metastasis of CD73+ tumors.Proc Natl Acad Sci U S A. 2013 Sep 3;110(36):14711-6. doi: 10.1073/pnas.1308209110. Epub 2013 Aug 20. Proc Natl Acad Sci U S A. 2013. PMID: 23964122 Free PMC article.
-
Ecto-5'-nucleotidase (CD73) attenuates allograft airway rejection through adenosine 2A receptor stimulation.J Immunol. 2010 Jul 15;185(2):1321-9. doi: 10.4049/jimmunol.0901847. Epub 2010 Jun 14. J Immunol. 2010. PMID: 20548026 Free PMC article.
-
CD73 has distinct roles in nonhematopoietic and hematopoietic cells to promote tumor growth in mice.J Clin Invest. 2011 Jun;121(6):2371-82. doi: 10.1172/JCI45559. Epub 2011 May 2. J Clin Invest. 2011. PMID: 21537079 Free PMC article.
-
CD73: a novel target for cancer immunotherapy.Cancer Res. 2010 Aug 15;70(16):6407-11. doi: 10.1158/0008-5472.CAN-10-1544. Epub 2010 Aug 3. Cancer Res. 2010. PMID: 20682793 Free PMC article. Review.
-
CD73-adenosine: a next-generation target in immuno-oncology.Immunotherapy. 2016 Feb;8(2):145-63. doi: 10.2217/imt.15.106. Epub 2016 Jan 25. Immunotherapy. 2016. PMID: 26808918 Review.
Cited by
-
Unraveling the Intricacies of CD73/Adenosine Signaling: The Pulmonary Immune and Stromal Microenvironment in Lung Cancer.Cancers (Basel). 2023 Dec 4;15(23):5706. doi: 10.3390/cancers15235706. Cancers (Basel). 2023. PMID: 38067409 Free PMC article. Review.
-
A Metabolic Immune Checkpoint: Adenosine in Tumor Microenvironment.Front Immunol. 2016 Mar 29;7:109. doi: 10.3389/fimmu.2016.00109. eCollection 2016. Front Immunol. 2016. PMID: 27066002 Free PMC article. Review.
-
Adenosine and cAMP signalling skew human dendritic cell differentiation towards a tolerogenic phenotype with defective CD8(+) T-cell priming capacity.Immunology. 2013 Apr;138(4):402-10. doi: 10.1111/imm.12053. Immunology. 2013. PMID: 23278551 Free PMC article.
-
Antagonism of adenosine A2A receptor expressed by lung adenocarcinoma tumor cells and cancer associated fibroblasts inhibits their growth.Cancer Biol Ther. 2013 Sep;14(9):860-8. doi: 10.4161/cbt.25643. Epub 2013 Jul 17. Cancer Biol Ther. 2013. PMID: 23917542 Free PMC article.
-
Adenosine Blockage in Tumor Microenvironment and Improvement of Cancer Immunotherapy.Immune Netw. 2019 Aug 27;19(4):e23. doi: 10.4110/in.2019.19.e23. eCollection 2019 Aug. Immune Netw. 2019. PMID: 31501711 Free PMC article. Review.
References
-
- Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–74. - PubMed
-
- Schreiber H, editor. Tumor Immunology. 5th ed. Lippincott-Williams & Wikins; Philadelphia, PA: 2003.
-
- Resta R, Yamashita Y, Thompson LF. Ecto-enzyme and signaling functions of lymphocyte CD73. Immunol Rev. 1998;161:95–109. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

