Vascular endothelial growth factor-C induces lymphangitic carcinomatosis, an extremely aggressive form of lung metastases
- PMID: 20179201
- PMCID: PMC4648266
- DOI: 10.1158/0008-5472.CAN-09-3675
Vascular endothelial growth factor-C induces lymphangitic carcinomatosis, an extremely aggressive form of lung metastases
Abstract
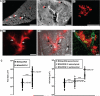
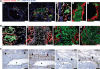
The lymphatic system is an important pathway for tumor dissemination to the lymph nodes, but to which extent it contributes to the formation of distant metastases remains unknown. We report that induction of lymphangiogenesis by vascular endothelial growth factor-C (VEGF-C) at the secondary site, in the lung, facilitates expansion of already disseminated cancer cells throughout the lung tissue. By using orthotopic spontaneous metastasis models in nude mice, we show that VEGF-C expression by tumor cells altered the pattern of pulmonary metastases from nodular to diffuse and facilitated disease progression. Metastases expressing VEGF-C were tightly associated with the airways, in contrast to the control cells that were scattered in the lung parenchyma, throughout the alveolar region. VEGF-C induced lung lymphangiogenesis and promoted intralymphatic spread of metastases in the lung and formation of tumor emboli in the pulmonary arteries. This pattern of metastasis corresponds to lymphangitic carcinomatosis metastatic phenotype in human cancer patients, an extremely aggressive pattern of pulmonary metastases. In accordance, pulmonary breast cancer metastases from patients which were classified as lymphangitic carcinomatosis showed high levels of VEGF-C expression in cancer cells. These data show that VEGF-C promotes late steps of the metastatic process and identify the VEGF-C/VEGF receptor-3 pathway as the target not only for prevention of metastases, but also for treatment of established metastatic disease.
Figures




References
-
- Tomashefski JF, Dail DH. Dail and Hammar's Pulmonary Pathology. 3rd ed. Springer; New York: 2008.
-
- Alitalo K, Carmeliet P. Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell. 2002;1:219–27. - PubMed
-
- Pepper MS, Tille JC, Nisato R, Skobe M. Lymphangiogenesis and tumor metastasis. Cell Tissue Res. 2003;314:167–77. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

