Activation of phosphatidylcholine cycle enzymes in human epithelial ovarian cancer cells
- PMID: 20179205
- PMCID: PMC2831129
- DOI: 10.1158/0008-5472.CAN-09-3833
Activation of phosphatidylcholine cycle enzymes in human epithelial ovarian cancer cells
Abstract
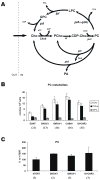
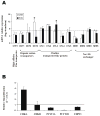
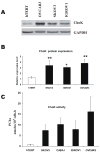
Altered phosphatidylcholine (PC) metabolism in epithelial ovarian cancer (EOC) could provide choline-based imaging approaches as powerful tools to improve diagnosis and identify new therapeutic targets. The increase in the major choline-containing metabolite phosphocholine (PCho) in EOC compared with normal and nontumoral immortalized counterparts (EONT) may derive from (a) enhanced choline transport and choline kinase (ChoK)-mediated phosphorylation, (b) increased PC-specific phospholipase C (PC-plc) activity, and (c) increased intracellular choline production by PC deacylation plus glycerophosphocholine-phosphodiesterase (GPC-pd) or by phospholipase D (pld)-mediated PC catabolism followed by choline phosphorylation. Biochemical, protein, and mRNA expression analyses showed that the most relevant changes in EOC cells were (a) 12-fold to 25-fold ChoK activation, consistent with higher protein content and increased ChoKalpha (but not ChoKbeta) mRNA expression levels; and (b) 5-fold to 17-fold PC-plc activation, consistent with higher, previously reported, protein expression. PC-plc inhibition by tricyclodecan-9-yl-potassium xanthate (D609) in OVCAR3 and SKOV3 cancer cells induced a 30% to 40% reduction of PCho content and blocked cell proliferation. More limited and variable sources of PCho could derive, in some EOC cells, from 2-fold to 4-fold activation of pld or GPC-pd. Phospholipase A2 activity and isoform expression levels were lower or unchanged in EOC compared with EONT cells. Increased ChoKalpha mRNA, as well as ChoK and PC-plc protein expression, were also detected in surgical specimens isolated from patients with EOC. Overall, we showed that the elevated PCho pool detected in EOC cells primarily resulted from upregulation/activation of ChoK and PC-plc involved in PC biosynthesis and degradation, respectively.
Conflict of interest statement
Figures



Similar articles
-
Phosphatidylcholine-specific phospholipase C activation in epithelial ovarian cancer cells.Cancer Res. 2008 Aug 15;68(16):6541-9. doi: 10.1158/0008-5472.CAN-07-6763. Cancer Res. 2008. PMID: 18701477
-
Alterations of choline phospholipid metabolism in ovarian tumor progression.Cancer Res. 2005 Oct 15;65(20):9369-76. doi: 10.1158/0008-5472.CAN-05-1146. Cancer Res. 2005. PMID: 16230400
-
Stimulation of phosphatidylcholine breakdown by thrombin and carbachol but not by tyrosine kinase receptor ligands in cells transfected with M1 muscarinic receptors. Rapid desensitization of phosphocholine-specific (PC) phospholipase D but sustained activity of PC-phospholipase C.J Biol Chem. 1992 Nov 15;267(32):22759-69. J Biol Chem. 1992. PMID: 1331066
-
Choline kinase: an important target for cancer.Curr Med Chem. 2006;13(10):1169-86. doi: 10.2174/092986706776360923. Curr Med Chem. 2006. PMID: 16719778 Review.
-
Phosphatidylcholine breakdown and signal transduction.Biochim Biophys Acta. 1994 Apr 14;1212(1):26-42. doi: 10.1016/0005-2760(94)90186-4. Biochim Biophys Acta. 1994. PMID: 8155724 Review.
Cited by
-
Glucose restriction induces cell death in parental but not in homeodomain-interacting protein kinase 2-depleted RKO colon cancer cells: molecular mechanisms and implications for tumor therapy.Cell Death Dis. 2013 May 23;4(5):e639. doi: 10.1038/cddis.2013.163. Cell Death Dis. 2013. PMID: 23703384 Free PMC article.
-
Associations between dietary intake of choline and betaine and lung cancer risk.PLoS One. 2013;8(2):e54561. doi: 10.1371/journal.pone.0054561. Epub 2013 Feb 1. PLoS One. 2013. PMID: 23383301 Free PMC article.
-
Lipid metabolic reprogramming in tumor microenvironment: from mechanisms to therapeutics.J Hematol Oncol. 2023 Sep 12;16(1):103. doi: 10.1186/s13045-023-01498-2. J Hematol Oncol. 2023. PMID: 37700339 Free PMC article. Review.
-
MALDI Mass Spectrometry Imaging Highlights Specific Metabolome and Lipidome Profiles in Salivary Gland Tumor Tissues.Metabolites. 2022 Jun 8;12(6):530. doi: 10.3390/metabo12060530. Metabolites. 2022. PMID: 35736462 Free PMC article.
-
Type E Botulinum Neurotoxin-Producing Clostridium butyricum Strains Are Aerotolerant during Vegetative Growth.mSystems. 2019 Apr 30;4(2):e00299-18. doi: 10.1128/mSystems.00299-18. eCollection 2019 Mar-Apr. mSystems. 2019. PMID: 31058231 Free PMC article.
References
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics. CA Cancer J Clin. 2008;58:71–96. - PubMed
-
- Negendank WG. Studies of human tumors by MRS: a review. NMR Biomed. 1992;5:303–24. - PubMed
-
- Podo F. Tumour phospholipid metabolism. Review article NMR Biomed. 1999;12:413–39. - PubMed
-
- Gillies RJ, Morse DL. In vivo magnetic resonance spectroscopy in cancer. Annu Rev Biomed Eng. 2005;7:287–326. - PubMed
-
- Glunde K, Ackerstaff E, Mori N, Jacobs MA, Bhujwalla ZM. Choline phospholipid metabolism in cancer: consequences for molecular pharmaceutical interventions. Mol Pharm. 2006;3:496–506. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

