Protein kinase Ciota is required for pancreatic cancer cell transformed growth and tumorigenesis
- PMID: 20179210
- PMCID: PMC2881466
- DOI: 10.1158/0008-5472.CAN-09-2684
Protein kinase Ciota is required for pancreatic cancer cell transformed growth and tumorigenesis
Abstract
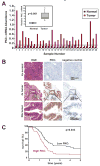
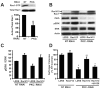
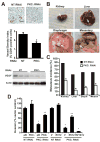
Pancreatic cancer is the fourth leading cause of cancer deaths in the United States, with an overall 5-year survival rate of <5%. Pancreatic ductal adenocarcinoma (PDAC), the most common form of pancreatic cancer, is highly resistant to conventional chemotherapies, underscoring the critical need for new molecular targets for pancreatic cancer chemotherapy. The KRAS proto-oncogene is mutated in >90% of PDAC. Protein kinase Ciota (PKCiota) is required for the oncogenic Ras-mediated transformed growth of lung cancer and intestinal epithelial cells. However, little is known about the role of PKCiota in pancreatic cancer. In this study, we evaluated the expression of PKCiota in human pancreatic cancer and the requirement for PKCiota for the transformed growth and tumorigenicity of PDAC cells. We find that PKCiota is significantly overexpressed in human pancreatic cancer, and high PKCiota expression correlates with poor patient survival. Inhibition of PKCiota expression blocks PDAC cell transformed growth in vitro and tumorigenicity in vivo. Inhibition of PKCiota expression in pancreatic tumors also significantly reduces tumor angiogenesis and metastasis. Analysis of downstream PKCiota effectors implicates the Rac1-MEK/ERK1/2 signaling axis in PKCiota-mediated transformed growth and cellular invasion. Taken together, our data show a required role for PKCiota in the transformed growth of pancreatic cancer cells and reveal a novel role for PKCiota in pancreatic cancer cell metastasis and angiogenesis in vivo. Our results strongly indicate that PKCiota will be an effective target for pancreatic cancer therapy.
Figures



References
-
- Lebedeva IV, Sarkar D, Su ZZ, et al. Molecular target-based therapy of pancreatic cancer. Cancer Res. 2006;66:2403–13. - PubMed
-
- Aoki K, Yoshida T, Matsumoto N, Ide H, Sugimura T, Terada M. Suppression of Ki-ras p21 levels leading to growth inhibition of pancreatic cancer cell lines with Ki-ras mutation but not those without Ki-ras mutation. Mol Carcinog. 1997;20:251–8. - PubMed
-
- Saad ED, Hoff PM. Molecular-targeted agents in pancreatic cancer. Cancer Control. 2004;11:32–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

