Toll-like receptor 4 modulates skeletal muscle substrate metabolism
- PMID: 20179247
- PMCID: PMC2867377
- DOI: 10.1152/ajpendo.00307.2009
Toll-like receptor 4 modulates skeletal muscle substrate metabolism
Abstract
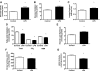
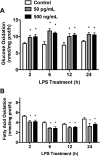
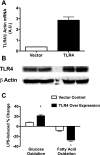
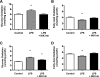
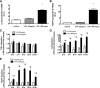
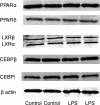
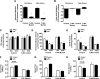
Toll-like receptor 4 (TLR4), a protein integral to innate immunity, is elevated in skeletal muscle of obese and type 2 diabetic humans and has been implicated in the development of lipid-induced insulin resistance. The purpose of this study was to examine the role of TLR4 as a modulator of basal (non-insulin-stimulated) substrate metabolism in skeletal muscle with the hypothesis that its activation would result in reduced fatty acid oxidation and increased partitioning of fatty acids toward neutral lipid storage. Human skeletal muscle, rodent skeletal muscle, and skeletal muscle cell cultures were employed to study the functional consequences of TLR4 activation on glucose and fatty acid metabolism. Herein, we demonstrate that activation of TLR4 with low (metabolic endotoxemia) and high (septic conditions) doses of LPS results in increased glucose utilization and reduced fatty acid oxidation in skeletal muscle and that these changes in metabolism in vivo occur in concert with increased circulating triglycerides. Moreover, animals with a loss of TLR4 function possess increased oxidative capacity in skeletal muscle and present with lower fasting levels of triglycerides and nonesterified free fatty acids. Evidence is also presented to suggest that these changes in substrate metabolism under metabolic endotoxemic conditions are independent of skeletal muscle-derived proinflammatory cytokine production. This report illustrates that skeletal muscle is a target for circulating endotoxin and may provide critical insight into the link between a proinflammatory state and dysregulated metabolism as observed with obesity, type 2 diabetes, and metabolic syndrome.
Figures
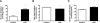
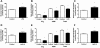
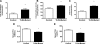
Similar articles
-
Elevated toll-like receptor 4 expression and signaling in muscle from insulin-resistant subjects.Diabetes. 2008 Oct;57(10):2595-602. doi: 10.2337/db08-0038. Epub 2008 Jul 15. Diabetes. 2008. PMID: 18633101 Free PMC article.
-
Activation of peroxisome proliferator-activated receptor-{delta} by GW501516 prevents fatty acid-induced nuclear factor-{kappa}B activation and insulin resistance in skeletal muscle cells.Endocrinology. 2010 Apr;151(4):1560-9. doi: 10.1210/en.2009-1211. Epub 2010 Feb 25. Endocrinology. 2010. PMID: 20185762
-
Overexpression of the orphan receptor Nur77 alters glucose metabolism in rat muscle cells and rat muscle in vivo.Diabetologia. 2010 Jun;53(6):1174-83. doi: 10.1007/s00125-010-1703-2. Epub 2010 Mar 9. Diabetologia. 2010. PMID: 20217038
-
Fuel selection in human skeletal muscle in insulin resistance: a reexamination.Diabetes. 2000 May;49(5):677-83. doi: 10.2337/diabetes.49.5.677. Diabetes. 2000. PMID: 10905472 Review.
-
Skeletal muscle triglyceride. An aspect of regional adiposity and insulin resistance.Diabetes Care. 2001 May;24(5):933-41. doi: 10.2337/diacare.24.5.933. Diabetes Care. 2001. PMID: 11347757 Review.
Cited by
-
Mex3c mutation reduces adiposity and increases energy expenditure.Mol Cell Biol. 2012 Nov;32(21):4350-62. doi: 10.1128/MCB.00452-12. Epub 2012 Aug 27. Mol Cell Biol. 2012. PMID: 22927639 Free PMC article.
-
Immunometabolism in type 2 diabetes mellitus: tissue-specific interactions.Arch Med Sci. 2020 Jan 31;19(4):895-911. doi: 10.5114/aoms.2020.92674. eCollection 2023. Arch Med Sci. 2020. PMID: 37560741 Free PMC article.
-
Mice lacking microRNA 133a develop dynamin 2–dependent centronuclear myopathy.J Clin Invest. 2011 Aug;121(8):3258-68. doi: 10.1172/JCI46267. J Clin Invest. 2011. PMID: 21737882 Free PMC article.
-
Dietary supplementation of chinese ginseng prevents obesity and metabolic syndrome in high-fat diet-fed mice.J Med Food. 2014 Dec;17(12):1287-97. doi: 10.1089/jmf.2014.0016. J Med Food. 2014. PMID: 25076190 Free PMC article.
-
Regulation of mitochondrial morphology and cristae architecture by the TLR4 pathway in human skeletal muscle.Front Cell Dev Biol. 2023 Jun 26;11:1212779. doi: 10.3389/fcell.2023.1212779. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37435031 Free PMC article.
References
-
- Al-Khalili L, Bouzakri K, Glund S, Lonnqvist F, Koistinen HA, Krook A. Signaling specificity of interleukin-6 action on glucose and lipid metabolism in skeletal muscle. Mol Endocrinol 20: 3364–3375, 2006 - PubMed
-
- Boden G. Fatty acid-induced inflammation and insulin resistance in skeletal muscle and liver. Curr Diab Rep 6: 177–181, 2006 - PubMed
-
- Boos CJ, Lip GY. Is hypertension an inflammatory process? Curr Pharm Des 12: 1623–1635, 2006 - PubMed
-
- Bruce CR, Dyck DJ. Cytokine regulation of skeletal muscle fatty acid metabolism: effect of interleukin-6 and tumor necrosis factor-α. Am J Physiol Endocrinol Metab 287: E616–E621, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

