Mice deficient in group VIB phospholipase A2 (iPLA2gamma) exhibit relative resistance to obesity and metabolic abnormalities induced by a Western diet
- PMID: 20179248
- PMCID: PMC2886524
- DOI: 10.1152/ajpendo.00780.2009
Mice deficient in group VIB phospholipase A2 (iPLA2gamma) exhibit relative resistance to obesity and metabolic abnormalities induced by a Western diet
Abstract
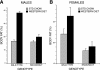
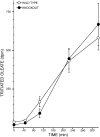
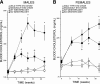
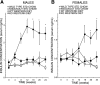
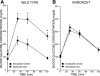
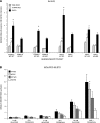
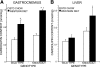
Phospholipases A(2) (PLA(2)) play important roles in metabolic processes, and the Group VI PLA(2) family is comprised of intracellular enzymes that do not require Ca(2+) for catalysis. Mice deficient in Group VIA PLA(2) (iPLA(2)beta) develop more severe glucose intolerance than wild-type (WT) mice in response to dietary stress. Group VIB PLA(2) (iPLA(2)gamma) is a related enzyme distributed in membranous organelles, including mitochondria, and iPLA(2)gamma knockout (KO) mice exhibit altered mitochondrial morphology and function. We have compared metabolic responses of iPLA(2)gamma-KO and WT mice fed a Western diet (WD) with a high fat content. We find that KO mice are resistant to WD-induced increases in body weight and adiposity and in blood levels of cholesterol, glucose, and insulin, even though WT and KO mice exhibit similar food consumption and dietary fat digestion and absorption. KO mice are also relatively resistant to WD-induced insulin resistance, glucose intolerance, and altered patterns of fat vs. carbohydrate fuel utilization. KO skeletal muscle exhibits impaired mitochondrial beta-oxidation of fatty acids, as reflected by accumulation of larger amounts of long-chain acylcarnitine (LCAC) species in KO muscle and liver compared with WT in response to WD feeding. This is associated with increased urinary excretion of LCAC and much reduced deposition of triacylglycerols in liver by WD-fed KO compared with WT mice. The iPLA(2)gamma-deficient genotype thus results in a phenotype characterized by impaired mitochondrial oxidation of fatty acids and relative resistance to the metabolic abnormalities induced by WD.
Figures


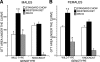









Similar articles
-
Genetic ablation of calcium-independent phospholipase A2gamma prevents obesity and insulin resistance during high fat feeding by mitochondrial uncoupling and increased adipocyte fatty acid oxidation.J Biol Chem. 2010 Nov 19;285(47):36495-510. doi: 10.1074/jbc.M110.115766. Epub 2010 Sep 3. J Biol Chem. 2010. PMID: 20817734 Free PMC article.
-
Glucose homeostasis, insulin secretion, and islet phospholipids in mice that overexpress iPLA2beta in pancreatic beta-cells and in iPLA2beta-null mice.Am J Physiol Endocrinol Metab. 2008 Feb;294(2):E217-29. doi: 10.1152/ajpendo.00474.2007. Epub 2007 Sep 25. Am J Physiol Endocrinol Metab. 2008. PMID: 17895289 Free PMC article.
-
Alleviation of high-fat diet-induced fatty liver damage in group IVA phospholipase A2-knockout mice.PLoS One. 2009 Dec 1;4(12):e8089. doi: 10.1371/journal.pone.0008089. PLoS One. 2009. PMID: 19956652 Free PMC article.
-
Insulin secretory responses and phospholipid composition of pancreatic islets from mice that do not express Group VIA phospholipase A2 and effects of metabolic stress on glucose homeostasis.J Biol Chem. 2006 Jul 28;281(30):20958-20973. doi: 10.1074/jbc.M600075200. Epub 2006 May 27. J Biol Chem. 2006. PMID: 16732058 Free PMC article.
-
Phospholipase A2 biochemistry.Cardiovasc Drugs Ther. 2009 Feb;23(1):49-59. doi: 10.1007/s10557-008-6132-9. Epub 2008 Oct 18. Cardiovasc Drugs Ther. 2009. PMID: 18931897 Free PMC article. Review.
Cited by
-
Metabolic Effects of Selective Deletion of Group VIA Phospholipase A2 from Macrophages or Pancreatic Islet Beta-Cells.Biomolecules. 2020 Oct 17;10(10):1455. doi: 10.3390/biom10101455. Biomolecules. 2020. PMID: 33080873 Free PMC article.
-
Lysophosphatidylcholine as an effector of fatty acid-induced insulin resistance.J Lipid Res. 2011 Jun;52(6):1234-1246. doi: 10.1194/jlr.M014787. Epub 2011 Mar 28. J Lipid Res. 2011. PMID: 21447485 Free PMC article.
-
Lipoquality control by phospholipase A2 enzymes.Proc Jpn Acad Ser B Phys Biol Sci. 2017;93(9):677-702. doi: 10.2183/pjab.93.043. Proc Jpn Acad Ser B Phys Biol Sci. 2017. PMID: 29129849 Free PMC article. Review.
-
Genetic Ablation of Calcium-independent Phospholipase A2γ Exacerbates Glomerular Injury in Adriamycin Nephrosis in Mice.Sci Rep. 2019 Nov 7;9(1):16229. doi: 10.1038/s41598-019-52834-x. Sci Rep. 2019. PMID: 31700134 Free PMC article.
-
Reign in the membrane: How common lipids govern mitochondrial function.Curr Opin Cell Biol. 2020 Apr;63:162-173. doi: 10.1016/j.ceb.2020.01.006. Epub 2020 Feb 24. Curr Opin Cell Biol. 2020. PMID: 32106003 Free PMC article. Review.
References
-
- Ahmed K, Tunaru S, Offermanns S. GPR109A, GPR109B and GPR81, a family of hydroxy-carboxylic acid receptors. Trends Pharmacol 30: 557– 62, 2009 - PubMed
-
- An J, Muoio DM, Shiota M, Fujimoto Y, Cline GW, Shulman GI, Koves TR, Stevens R, Millington D, Newgard CB. Hepatic expression of malonyl-CoA decarboxylase reverses muscle, liver and whole-animal insulin resistance. Nat Med 10: 268– 274, 2004 - PubMed
-
- Andreyev AY, Kushnareva YE, Starkov AA. Mitochondrial metabolism of reactive oxygen species. Biochemistry (Mosc) 70: 200– 214, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

