Apoptotic cells activate the "phoenix rising" pathway to promote wound healing and tissue regeneration
- PMID: 20179271
- PMCID: PMC2905599
- DOI: 10.1126/scisignal.2000634
Apoptotic cells activate the "phoenix rising" pathway to promote wound healing and tissue regeneration
Abstract
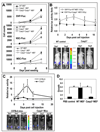
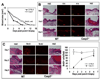
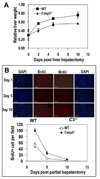
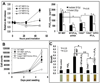
The ability to regenerate damaged tissues is a common characteristic of multicellular organisms. We report a role for apoptotic cell death in promoting wound healing and tissue regeneration in mice. Apoptotic cells released growth signals that stimulated the proliferation of progenitor or stem cells. Key players in this process were caspases 3 and 7, proteases activated during the execution phase of apoptosis that contribute to cell death. Mice lacking either of these caspases were deficient in skin wound healing and in liver regeneration. Prostaglandin E(2), a promoter of stem or progenitor cell proliferation and tissue regeneration, acted downstream of the caspases. We propose to call the pathway by which executioner caspases in apoptotic cells promote wound healing and tissue regeneration in multicellular organisms the "phoenix rising" pathway.
Figures

References
-
- Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. - PubMed
-
- Slack JM, Savage S. Regeneration of mirror symmetrical limbs in the axolotl. Cell. 1978;14:1–8. - PubMed
-
- Brockes JP, Kumar A. Plasticity and reprogramming of differentiated cells in amphibian regeneration. Nat Rev Mol Cell Biol. 2002;3:566–574. - PubMed
-
- Sanchez Alvarado A. Planarian regeneration: its end is its beginning. Cell. 2006;124:241–245. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

