Role of endosomal membrane lipids and NPC2 in cholesterol transfer and membrane fusion
- PMID: 20179319
- PMCID: PMC2882726
- DOI: 10.1194/jlr.M003822
Role of endosomal membrane lipids and NPC2 in cholesterol transfer and membrane fusion
Abstract
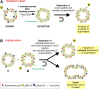
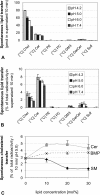
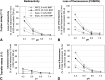
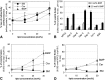
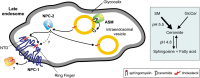
We examined the effect of Niemann-Pick disease type 2 (NPC2) protein and some late endosomal lipids [sphingomyelin, ceramide and bis(monoacylglycero)phosphate (BMP)] on cholesterol transfer and membrane fusion. Of all lipid-binding proteins tested, only NPC2 transferred cholesterol at a substantial rate, with no transfer of ceramide, GM3, galactosylceramide, sulfatide, phosphatidylethanolamine, or phosphatidylserine. Cholesterol transfer was greatly stimulated by BMP, little by ceramide, and strongly inhibited by sphingomyelin. Cholesterol and ceramide were also significantly transferred in the absence of protein. This spontaneous transfer of cholesterol was greatly enhanced by ceramide, slightly by BMP, and strongly inhibited by sphingomyelin. In our transfer assay, biotinylated donor liposomes were separated from fluorescent acceptor liposomes by streptavidin-coated magnetic beads. Thus, the loss of fluorescence indicated membrane fusion. Ceramide induced spontaneous fusion of lipid vesicles even at very low concentrations, while BMP and sphingomyelin did so at about 20 mol% and 10 mol% concentrations, respectively. In addition to transfer of cholesterol, NPC2 induced membrane fusion, although less than saposin-C. In this process, BMP and ceramide had a strong and mild stimulating effect, and sphingomyelin an inhibiting effect, respectively. Note that the effects of the lipids on cholesterol transfer mediated by NPC2 were similar to their effect on membrane fusion induced by NPC2 and saposin-C.
Figures




References
-
- Brown M. S., Goldstein J. L. 1986. A receptor-mediated pathway for cholesterol homeostasis. Science. 232: 34–47. - PubMed
-
- Ikonen E. 2008. Cellular cholesterol trafficking and compartmentalization. Nat. Rev. Mol. Cell Biol. 9: 125–138. - PubMed
-
- Möbius W., van Donselaar E., Ohno-Iwashita Y., Shimada Y., Heijnen H. F., Slot J. W., Geuze H. J. 2003. Recycling compartments and the internal vesicles of multivesicular bodies harbor most of the cholesterol found in the endocytic pathway. Traffic. 4: 222–231. - PubMed
-
- Pentchev P. G., Comly M. E., Kruth H. S., Tokoro T., Butler J., Sokol J., Filling-Katz M., Quirk J. M., Marshall D. C., Patel S., et al. 1987. Group C Niemann-Pick disease: faulty regulation of low-density lipoprotein uptake and cholesterol storage in cultured fibroblasts. FASEB J. 1: 40–45. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

