Structure of the unbound form of HIV-1 subtype A protease: comparison with unbound forms of proteases from other HIV subtypes
- PMID: 20179334
- PMCID: PMC2827345
- DOI: 10.1107/S0907444909054298
Structure of the unbound form of HIV-1 subtype A protease: comparison with unbound forms of proteases from other HIV subtypes
Abstract
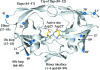
The crystal structure of the unbound form of HIV-1 subtype A protease (PR) has been determined to 1.7 A resolution and refined as a homodimer in the hexagonal space group P6(1) to an R(cryst) of 20.5%. The structure is similar in overall shape and fold to the previously determined subtype B, C and F PRs. The major differences lie in the conformation of the flap region. The flaps in the crystal structures of the unbound subtype B and C PRs, which were crystallized in tetragonal space groups, are either semi-open or wide open. In the present structure of subtype A PR the flaps are found in the closed position, a conformation that would be more anticipated in the structure of HIV protease complexed with an inhibitor. The amino-acid differences between the subtypes and their respective crystal space groups are discussed in terms of the differences in the flap conformations.
Figures





References
-
- Abecasis, A. B., Deforche, K., Bacheler, L. T., McKenna, P., Carvalho, A. P., Gomes, P., Vandamme, A. M. & Camacho, R. (2006). J. Antivir. Ther.11, 581–589. - PubMed
-
- Baldanti, F., Paolucci, S., Ravasi, G., Maccabruni, A., Moriggia, A., Barbarini, G. & Maserati, R. (2008). J. Med. Virol.80, 947–952. - PubMed
-
- Brodine, S. K., Mascola, J. R., Weiss, P. J., Ito, S. I., Porter, K. R., Artenstein, A. W., Garland, F. C., McCutchan, F. E. & Burke, D. S. (1995). Lancet, 346, 1198–1199. - PubMed
-
- Brünger, A. T., Adams, P. D., Clore, G. M., DeLano, W. L., Gros, P., Grosse-Kunstleve, R. W., Jiang, J.-S., Kuszewski, J., Nilges, M., Pannu, N. S., Read, R. J., Rice, L. M., Simonson, T. & Warren, G. L. (1998). Acta Cryst. D54, 905–921. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

