Rapid model building of alpha-helices in electron-density maps
- PMID: 20179338
- PMCID: PMC2827347
- DOI: 10.1107/S0907444910000314
Rapid model building of alpha-helices in electron-density maps
Abstract
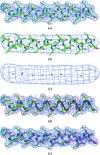
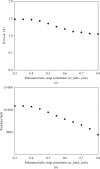
A method for the identification of alpha-helices in electron-density maps at low resolution followed by interpretation at moderate to high resolution is presented. Rapid identification is achieved at low resolution, where alpha-helices appear as tubes of density. The positioning and direction of the alpha-helices is obtained at moderate to high resolution, where the positions of side chains can be seen. The method was tested on a set of 42 experimental electron-density maps at resolutions ranging from 1.5 to 3.8 A. An average of 63% of the alpha-helical residues in these proteins were built and an average of 76% of the residues built matched helical residues in the refined models of the proteins. The overall average r.m.s.d. between main-chain atoms in the modeled alpha-helices and the nearest atom with the same name in the refined models of the proteins was 1.3 A.
Figures

References
-
- Adams, P. D., Grosse-Kunstleve, R. W., Hung, L.-W., Ioerger, T. R., McCoy, A. J., Moriarty, N. W., Read, R. J., Sacchettini, J. C., Sauter, N. K. & Terwilliger, T. C. (2002). Acta Cryst. D58, 1948–1954. - PubMed
-
- Alphey, M. S., Leonard, G. A., Gourley, D. G., Tetaud, E., Fairlamb, A. H. & Hunter, W. N. (1999). J. Biol. Chem. 274, 25613–25622. - PubMed
-
- Bernstein, F. C., Koetzle, T. F., Williams, G. J. B., Meyer, E. F. Jr, Brice, M. D., Rodgers, J. R., Kennard, O., Shimanouchi, T. & Tasumi, M. (1977). J. Mol. Biol. 112, 535–542. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

