Activated regulatory T cells are the major T cell type emigrating from the skin during a cutaneous immune response in mice
- PMID: 20179354
- PMCID: PMC2827959
- DOI: 10.1172/JCI40926
Activated regulatory T cells are the major T cell type emigrating from the skin during a cutaneous immune response in mice
Abstract
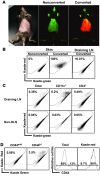
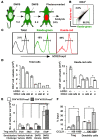
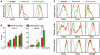
Tregs play an important role in protecting the skin from autoimmune attack. However, the extent of Treg trafficking between the skin and draining lymph nodes (DLNs) is unknown. We set out to investigate this using mice engineered to express the photoconvertible fluorescence protein Kaede, which changes from green to red when exposed to violet light. By exposing the skin of Kaede-transgenic mice to violet light, we were able to label T cells in the periphery under physiological conditions with Kaede-red and demonstrated that both memory phenotype CD4+Foxp3- non-Tregs and CD4+Foxp3+ Tregs migrated from the skin to DLNs in the steady state. During cutaneous immune responses, Tregs constituted the major emigrants and inhibited immune responses more robustly than did LN-resident Tregs. We consistently observed that cutaneous immune responses were prolonged by depletion of endogenous Tregs in vivo. In addition, the circulating Tregs specifically included activated CD25hi Tregs that demonstrated a strong inhibitory function. Together, our results suggest that Tregs in circulation infiltrate the periphery, traffic to DLNs, and then recirculate back to the skin, contributing to the downregulation of cutaneous immune responses.
Figures




Comment in
-
Bidirectional homing of Tregs between the skin and lymph nodes.J Clin Invest. 2010 Mar;120(3):653-6. doi: 10.1172/JCI42280. Epub 2010 Feb 22. J Clin Invest. 2010. PMID: 20179349 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

