Endothelial dysfunction enhances vasoconstriction due to scavenging of nitric oxide by a hemoglobin-based oxygen carrier
- PMID: 20179495
- PMCID: PMC2829726
- DOI: 10.1097/ALN.0b013e3181cd7838
Endothelial dysfunction enhances vasoconstriction due to scavenging of nitric oxide by a hemoglobin-based oxygen carrier
Abstract
Background: To date, there is no safe and effective hemoglobin-based oxygen carrier (HBOC) to substitute for erythrocyte transfusion. It is uncertain whether a deficiency of endothelial nitric oxide bioavailability (endothelial dysfunction) prevents or augments HBOC-induced vasoconstriction.
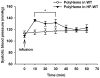
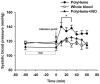
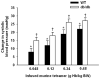
Methods: Hemodynamic effects of infusion of PolyHeme (1.08 g hemoglobin/kg; Northfield Laboratories, Evanston, IL) or murine tetrameric hemoglobin (0.48 g hemoglobin/kg) were determined in awake healthy lambs, awake mice, and anesthetized mice. In vitro, a cumulative dose-tension response was obtained by sequential addition of PolyHeme or tetrameric hemoglobin to phenylephrine-precontracted murine aortic rings.
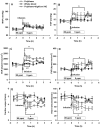
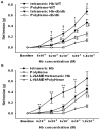
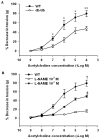
Results: Infusion of PolyHeme did not cause systemic hypertension in awake lambs but produced acute systemic and pulmonary vasoconstriction. Infusion of PolyHeme did not cause systemic hypertension in healthy wild-type mice but induced severe systemic vasoconstriction in mice with endothelial dysfunction (either db/db mice or high-fat fed wild-type mice for 4-6 weeks). The db/db mice were more sensitive to systemic vasoconstriction than wild-type mice after the infusion of either tetrameric hemoglobin or PolyHeme. Murine aortic ring studies confirmed that db/db mice have an impaired response to an endothelial-dependent vasodilator and an enhanced vasoconstrictor response to HBOC.
Conclusions: Reduction in low molecular weight hemoglobin concentrations to less than 1% is insufficient to abrogate the vasoconstrictor effects of HBOC infusion in healthy awake sheep or in mice with reduced vascular nitric oxide levels associated with endothelial dysfunction. These findings suggest that testing HBOCs in animals with endothelial dysfunction can provide a more sensitive indication of their potential vasoconstrictor effects.
Conflict of interest statement
Conflicts of Interest
Dr. Zapol receives royalties on patents licensed by Massachusetts General Hospital to Linde Corporation, Munich, Germany, and Ikaria, Clinton, New Jersey, on inhaled nitric oxide. Dr. Bloch has received grants from Ikaria and Linde to study inhaled nitric oxide. Northfield Corp. (Evanston, IL) funded the animal charges and provided the PolyHeme at no cost. The remaining authors report no conflicts of interest.
Figures
References
-
- Moore EE, Moore FA, Fabian TC, Bernard AC, Fulda GJ, Hoyt DB, Duane TM, Weireter LJ, Jr, Gomez GA, Cipolle MD, Rodman GH, Jr, Malangoni MA, Hides GA, Omert LA, Gould SA. PolyHeme Study Group: Human polymerized hemoglobin for the treatment of hemorrhagic shock when blood is unavailable: the USA multicenter trial. J Am Coll Surg. 2009;208:1–13. - PubMed
-
- Reiter CD, Wang X, Tanus-Santos JE, Hogg N, Cannon RO, III, Schechter AN, Gladwin MT. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 2002;8:1383–9. - PubMed
-
- Gladwin MT, Sachdev V, Jison MJ, Shizukuda Y, Plehn JF, Minter K, Brown B, Coles WA, Nichols JS, Ernst I, Hunter LA, Blackwelder WC, Schechter AN, Rodgers GP, Castro O, Ognibene FP. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350:886–95. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous