In vivo delivery of antigens by adenovirus dodecahedron induces cellular and humoral immune responses to elicit antitumor immunity
- PMID: 20179681
- PMCID: PMC2890114
- DOI: 10.1038/mt.2010.16
In vivo delivery of antigens by adenovirus dodecahedron induces cellular and humoral immune responses to elicit antitumor immunity
Abstract
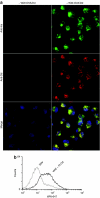
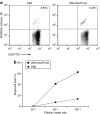
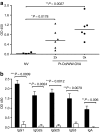
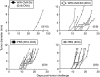
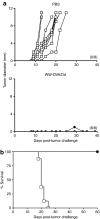
Cancer vaccines based on virus-like particles (VLPs) vectors may offer many advantages over other antigen-delivery systems and represent an alternative to the ex vivo cell therapy approach. In this study, we describe the use of penton-dodecahedron (Pt-Dd) VLPs from human adenovirus type 3 (Ad3) as cancer vaccine vehicle for specific antigens, based on its unique cellular internalization properties. WW domains from the ubiquitin ligase Nedd4 serve as an adapter to bind the antigen to Pt-Dd. By engineering fusion partners of WW with the model antigen ovalbumin (OVA), Pt-Dd can efficiently deliver WW-OVA in vitro and the Pt-Dd/WW complex can be readily internalized by dendritic cells (DCs). Immunization with WW-OVA/Pt-Dd results in 90% protection against B16-OVA melanoma implantation in syngeneic mice. This high level of protection correlates with the development of OVA-specific CD8(+) T cells. Moreover, vaccination with WW-OVA Pt-Dd induces robust humoral responses in mice as shown by the high levels of anti-OVA antibodies (Abs) detected in serum. Importantly, treatment of mice bearing B16-OVA tumors with WW-OVA/Pt-Dd results in complete tumor regression in 100% of cases. Thus, our data supports a dual role of Pt-Dd as antigen-delivery vector and natural adjuvant, able to generate integrated cellular and humoral responses of broad immunogenic complexity to elicit specific antitumor immunity. Antigen delivery by Pt-Dd vector is a promising novel strategy for development of cancer vaccines with important clinical applications.
Figures


Similar articles
-
Efficient antigen gene transduction using Arg-Gly-Asp fiber-mutant adenovirus vectors can potentiate antitumor vaccine efficacy and maturation of murine dendritic cells.Cancer Res. 2001 Nov 1;61(21):7913-9. Cancer Res. 2001. PMID: 11691812
-
Pathogen-mimicking vaccine delivery system designed with a bioactive polymer (inulin acetate) for robust humoral and cellular immune responses.J Control Release. 2017 Sep 10;261:263-274. doi: 10.1016/j.jconrel.2017.06.026. Epub 2017 Jun 29. J Control Release. 2017. PMID: 28669593 Free PMC article.
-
Evaluation of pH-sensitive fusogenic polymer-modified liposomes co-loaded with antigen and α-galactosylceramide as an anti-tumor vaccine.J Vet Med Sci. 2018 Feb 9;80(2):197-204. doi: 10.1292/jvms.17-0491. Epub 2017 Dec 28. J Vet Med Sci. 2018. PMID: 29311431 Free PMC article.
-
Cell entry and innate sensing shape adaptive immune responses to adenovirus-based vaccines.Curr Opin Immunol. 2023 Feb;80:102282. doi: 10.1016/j.coi.2023.102282. Epub 2023 Jan 28. Curr Opin Immunol. 2023. PMID: 36716578 Review.
-
Sustained delivery approaches to improving adaptive immune responses.Adv Drug Deliv Rev. 2022 Aug;187:114401. doi: 10.1016/j.addr.2022.114401. Epub 2022 Jun 21. Adv Drug Deliv Rev. 2022. PMID: 35750115 Review.
Cited by
-
Genome-wide analysis of the WW domain-containing protein genes in silkworm and their expansion in eukaryotes.Mol Genet Genomics. 2015 Jun;290(3):807-24. doi: 10.1007/s00438-014-0958-6. Epub 2014 Nov 26. Mol Genet Genomics. 2015. PMID: 25424044
-
The Use of Adenovirus Dodecahedron in the Delivery of an Enzymatic Activity in the Cell.Biotechnol Res Int. 2016;2016:5030589. doi: 10.1155/2016/5030589. Epub 2016 May 8. Biotechnol Res Int. 2016. PMID: 27242929 Free PMC article.
-
Functional characterisation of the WW minimal domain for delivering therapeutic proteins by adenovirus dodecahedron.PLoS One. 2012;7(9):e45416. doi: 10.1371/journal.pone.0045416. Epub 2012 Sep 27. PLoS One. 2012. PMID: 23028993 Free PMC article.
-
The structural basis for the integrity of adenovirus Ad3 dodecahedron.PLoS One. 2012;7(9):e46075. doi: 10.1371/journal.pone.0046075. Epub 2012 Sep 25. PLoS One. 2012. PMID: 23049939 Free PMC article.
-
The Adenovirus Dodecahedron: Beyond the Platonic Story.Viruses. 2020 Jul 2;12(7):718. doi: 10.3390/v12070718. Viruses. 2020. PMID: 32630840 Free PMC article. Review.
References
-
- Tacken PJ, de Vries IJ, Torensma R., and , Figdor CG. Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nat Rev Immunol. 2007;7:790–802. - PubMed
-
- Epaulard O, Toussaint B, Quenee L, Derouazi M, Bosco N, Villiers C, et al. Anti-tumor immunotherapy via antigen delivery from a live attenuated genetically engineered Pseudomonas aeruginosa type III secretion system-based vector. Mol Ther. 2006;14:656–661. - PubMed
-
- Radford KJ, Higgins DE, Pasquini S, Cheadle EJ, Carta L, Jackson AM, et al. A recombinant E. coli vaccine to promote MHC class I-dependent antigen presentation: application to cancer immunotherapy. Gene Ther. 2002;9:1455–1463. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

