Verapamil enhances the antitumoral efficacy of oncolytic adenoviruses
- PMID: 20179683
- PMCID: PMC2890100
- DOI: 10.1038/mt.2010.22
Verapamil enhances the antitumoral efficacy of oncolytic adenoviruses
Abstract
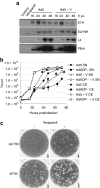
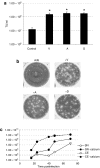
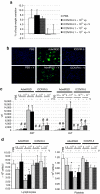
The therapeutic potential of oncolytic adenoviruses is limited by the rate of adenovirus release. Based on the observation that several viruses induce cell death and progeny release by disrupting intracellular calcium homeostasis, we hypothesized that the alteration in intracellular calcium concentration induced by verapamil could improve the rate of virus release and spread, eventually enhancing the antitumoral activity of oncolytic adenoviruses. Our results indicate that verapamil substantially enhanced the release of adenovirus from a variety of cell types resulting in an improved cell-to-cell spread and cytotoxicity. Furthermore, the combination of the systemic administration of an oncolytic adenovirus (ICOVIR-5) with verapamil in vivo greatly improved its antitumoral activity in two different tumor xenograft models without affecting the selectivity of this virus. Overall, our findings indicate that verapamil provides a new, safe, and versatile way to improve the antitumoral potency of oncolytic adenoviruses in the clinical setting.
Figures



References
-
- Liu TC, Galanis E., and , Kirn D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nat Clin Pract Oncol. 2007;4:101–117. - PubMed
-
- Alemany R, Suzuki K., and , Curiel DT. Blood clearance rates of adenovirus type 5 in mice. J Gen Virol. 2000;81 Pt 11:2605–2609. - PubMed
-
- Alemany R. Cancer selective adenoviruses. Mol Aspects Med. 2007;28:42–58. - PubMed
-
- Wein LM, Wu JT., and , Kirn DH. Validation and analysis of a mathematical model of a replication-competent oncolytic virus for cancer treatment: implications for virus design and delivery. Cancer Res. 2003;63:1317–1324. - PubMed
-
- Kim JH, Lee YS, Kim H, Huang JH, Yoon AR., and , Yun CO. Relaxin expression from tumor-targeting adenoviruses and its intratumoral spread, apoptosis induction, and efficacy. J Natl Cancer Inst. 2006;98:1482–1493. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

