Treatment of HER2-positive metastatic breast cancer with lapatinib and capecitabine in the lapatinib expanded access programme, including efficacy in brain metastases--the UK experience
- PMID: 20179708
- PMCID: PMC2844035
- DOI: 10.1038/sj.bjc.6605586
Treatment of HER2-positive metastatic breast cancer with lapatinib and capecitabine in the lapatinib expanded access programme, including efficacy in brain metastases--the UK experience
Abstract
Background: The global lapatinib expanded access programme provided access to lapatinib combined with capecitabine for women with HER2-positive metastatic breast cancer (MBC) who previously received anthracycline, taxane and trastuzumab.
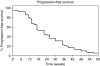
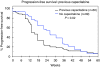
Methods: Progression-free survival (PFS) and safety data for 356 patients recruited from the United Kingdom are reported. Efficacy was assessed in 162 patients from the five lead centres, including objective tumour response rate (ORR), time to disease progression (TTP) and efficacy in those with central nervous system (CNS) metastases. Correlation of PFS and ORR with previous capecitabine treatment was also documented.
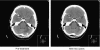
Results: Overall, PFS for the 356 UK patients was 21 weeks (95% CI: 17.6-24.7). In the 162 assessable patients, ORR was 21% (95% CI: 15-27%) and median TTP was 22 weeks (95% CI: 17-27). Efficacy was greater in capecitabine-naive patients (ORR 23 vs 16.3%, P=0.008). For 34 patients with CNS metastases, ORR was 21% (95% CI: 9-39%), with evidence of improvement in neurological symptoms, and median TTP was 22 weeks (95% CI: 15-28).
Conclusions: Lapatinib combined with capecitabine is an active treatment option for women with refractory HER2-positive MBC, including those with progressive CNS disease.
Figures



References
-
- Bendell JC, Domchek SM, Burstein HJ, Harris L, Younger J, Kuter I 2003) Central nervous system metastases in women who received trastuzumab based therapy for metastatic breast carcinoma. Cancer 97(12): 2972–2977 - PubMed
-
- Boccardo F, Kauffman B, Baselga J, Dieras V, Link J, Casey M, Fittipaldo A, Oliva C, Zembryki D, Rubin S (2008) Evaluation of lapatinib plus capecitabine in patients with brain metastases from Her2+ breast cancer enrolled in the expanded access program (LEAP) and French authorisation temporaire d’utilisation (ATU). J Clin Oncol (Meeting Abstracts) 26: 64s (Abstr 1094)
-
- Cameron D, Casey M, Press M, Lindquist D, Pienkowski T, Romieu CG, Chan S, Jagiello-Gruszfeld A, Kaufman B, Crown J, Chan A, Campone M, Viens P, Davidson N, Gorbounova V, Raats JI, Skarlos D, Newstat B, Roychowdhury D, Paoletti P, Oliva C, Rubin S, Stein S, Geyer CE (2008) A Phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analysis. Breast Cancer Res Treat 112(3): 533–543 - PubMed
-
- Capri G, Jerusalem G, Chang J, Jiang Z, Link J, Chen SC, Ro JS, De Placido S, Conte PF, Kaufman B, Johnston S, Schütte HJ, Cwiertka K, Preston A, Selzer M, Rosenlund J, Zembryki D, Oliva C, Parikh R (2009) An open-label expanded access study of lapatinib and capecitabine in patients with ErbB2-overexpressing locally advanced or metastatic breast cancer. Ann Oncol; E-pub ahead of print 2 November 2009. Advance access. doi:10.1093/annonc/mdp373 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

