Mapping the face in the somatosensory brainstem
- PMID: 20179712
- PMCID: PMC3545448
- DOI: 10.1038/nrn2804
Mapping the face in the somatosensory brainstem
Abstract
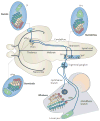
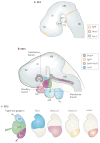
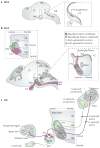
The facial somatosensory map in the cortex is derived from facial representations that are first established at the brainstem level and then serially 'copied' at each stage of the somatosensory pathway. Recent studies have provided insights into the molecular mechanisms involved in the development of somatotopic maps of the face and whiskers in the trigeminal nuclei of the mouse brainstem. This work has revealed that early molecular regionalization and positional patterning of trigeminal ganglion and brainstem target neurons are established by homeodomain transcription factors, the expression of which is induced and maintained by signals from the brain and face. Such position-dependent information is fundamental in transforming the early spatial layout of sensory receptors into a topographic connectivity map that is conferred to higher brain levels.
Conflict of interest statement
Competing interests statement
The authors declare no competing financial interests.
Figures

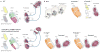
References
-
- Penfield W, Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937;60:389–443.
-
- Belford GR, Killackey HP. The development of vibrissae representation in subcortical trigeminal centers of the neonatal rat. J Comp Neurol. 1979;188:63–74. - PubMed
-
- Ma PM. The barrelettes — architectonic vibrissal representations in the brainstem trigeminal complex of the mouse. I Normal structural organization. J Comp Neurol. 1991;309:161–199. - PubMed
-
- Ma PM. Barrelettes — architectonic vibrissal representations in the brainstem trigeminal complex of the mouse. II Normal post-natal development. J Comp Neurol. 1993;327:376–397. - PubMed
-
- Ma PM, Woolsey TA. Cytoarchitectonic correlates of the vibrissae in the medullary trigeminal complex of the mouse. Brain Res. 1984;306:349–374. This report coined the term ‘barrelettes’ for the whisker-specific neural modules. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

