Retinal afferents synapse with relay cells targeting the middle temporal area in the pulvinar and lateral geniculate nuclei
- PMID: 20179789
- PMCID: PMC2826187
- DOI: 10.3389/neuro.05.008.2010
Retinal afferents synapse with relay cells targeting the middle temporal area in the pulvinar and lateral geniculate nuclei
Abstract
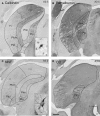
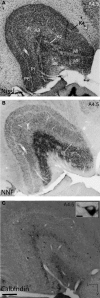
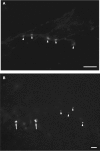
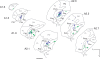
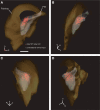
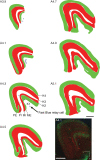
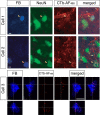
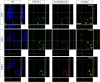
Considerable debate continues regarding thalamic inputs to the middle temporal area (MT) of the visual cortex that bypass the primary visual cortex (V1) and the role they might have in the residual visual capability following a lesion of V1. Two specific retinothalamic projections to area MT have been speculated to relay through the medial portion of the inferior pulvinar nucleus (PIm) and the koniocellular layers of the dorsal lateral geniculate nucleus (LGN). Although a number of studies have demonstrated retinal inputs to regions of the thalamus where relays to area MT have been observed, the relationship between the retinal terminals and area MT relay cells has not been established. Here we examined direct retino-recipient regions of the marmoset monkey (Callithrix jacchus) pulvinar nucleus and the LGN following binocular injections of anterograde tracer, as well as area MT relay cells in these nuclei by injection of retrograde tracer into area MT. Retinal afferents were shown to synapse with area MT relay cells as demonstrated by colocalization with the presynaptic vesicle membrane protein synaptophysin. We also established the presence of direct synapes of retinal afferents on area MT relay cells within the PIm, as well as the koniocellular K1 and K3 layers of the LGN, thereby corroborating the existence of two disynaptic pathways from the retina to area MT that bypass V1.
Keywords: V1; calcium-binding protein; extrastriate areas; neuronal tracing; nonhuman primate; synapse; synaptophysin; thalamus.
Figures

References
LinkOut - more resources
Full Text Sources

