Preparation, physicochemical characterization and cytotoxicity in vitro of gemcitabine-loaded PEG-PDLLA nanovesicles
- PMID: 20180242
- PMCID: PMC2828587
- DOI: 10.3748/wjg.v16.i8.1008
Preparation, physicochemical characterization and cytotoxicity in vitro of gemcitabine-loaded PEG-PDLLA nanovesicles
Abstract
Aim: To investigate the preparation, physicochemical characterization and cytotoxicity in vitro of Gemcitabine-loaded poly(ethylene glycol)-block-poly(D,L-lactide) (PEG-PDLLA) nanovesicles.
Methods: The nanovesicle carriers were prepared from the amphiphilic block copolymer of PEG-PDLLA by a double emulsion technique, and gemcitabine was used as the model drug. The morphology of the nanovesicles was determined by scanning and transmission electron microscopy, and the drug content, drug entrapment and drug-release curve in vitro were detected by UV-Vis-NIR spectrophotometry. Cytotoxicity in the human pancreatic cancer cell line SW1990 was tested by 3-(4,5-dimethyl) ethiazole (MTT) assay.
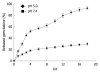
Results: The gemcitabine-loaded nanovesicles were hollow nanospheres with a mean size of 200.6 nm, drug loading of 4.14% and drug embedding ratio of 20.54%. The nanovesicles showed excellent controlled release that was characterized by a fast initial release during the first 72 h, followed by a slower and continuous release. The MTT assay demonstrated that gemcitabine-loaded nanovesicles exhibited dose-dependent and time-delayed cytotoxicity in the human pancreatic cancer cell line SW1990.
Conclusion: Gemcitabine-loaded PEG-PDLLA nanovesicles prepared by a double emulsion technique exhibited good performance for controlled drug release, and had similar cytotoxic activity to free gemcitabine.
Figures



Similar articles
-
Tailoring the properties of mPEG-PLLA nanoparticles for better encapsulation and tuned release of the hydrophilic anticancer drug.Drug Deliv Transl Res. 2017 Jun;7(3):416-427. doi: 10.1007/s13346-017-0372-9. Drug Deliv Transl Res. 2017. PMID: 28324320
-
[Investigation into the controlled release behavior of gemcitabine-loaded nanovesicles].Zhonghua Yi Xue Za Zhi. 2008 Jul 22;88(28):2005-7. Zhonghua Yi Xue Za Zhi. 2008. PMID: 19062746 Chinese.
-
Preparation, optimization and in vitro characterization of stearoyl-gemcitabine polymeric micelles: a comparison with its self-assembled nanoparticles.Int J Pharm. 2014 Jul 1;468(1-2):142-51. doi: 10.1016/j.ijpharm.2014.04.021. Epub 2014 Apr 13. Int J Pharm. 2014. PMID: 24731731
-
Effect of PEG-PDLLA polymeric nanovesicles loaded with doxorubicin and hematoporphyrin monomethyl ether on human hepatocellular carcinoma HepG2 cells in vitro.Int J Nanomedicine. 2013;8:4613-22. doi: 10.2147/IJN.S54142. Epub 2013 Dec 2. Int J Nanomedicine. 2013. PMID: 24324333 Free PMC article.
-
Folate-conjugated amphiphilic hyperbranched block copolymers based on Boltorn H40, poly(L-lactide) and poly(ethylene glycol) for tumor-targeted drug delivery.Biomaterials. 2009 Jun;30(16):3009-19. doi: 10.1016/j.biomaterials.2009.02.011. Epub 2009 Feb 27. Biomaterials. 2009. PMID: 19250665
Cited by
-
Tailoring the properties of mPEG-PLLA nanoparticles for better encapsulation and tuned release of the hydrophilic anticancer drug.Drug Deliv Transl Res. 2017 Jun;7(3):416-427. doi: 10.1007/s13346-017-0372-9. Drug Deliv Transl Res. 2017. PMID: 28324320
-
Encapsulating a Hydrophilic Chemotherapeutic into Rod-like Nanoparticles of a Genetically Encoded Asymmetric Triblock Polypeptide Improves its Efficacy.Adv Funct Mater. 2017 Mar 24;27(12):1605421. doi: 10.1002/adfm.201605421. Epub 2017 Feb 7. Adv Funct Mater. 2017. PMID: 30319320 Free PMC article.
-
Synthesis of Gemcitabine-Loaded PLGA Microparticles with Green Solvents.ACS Omega. 2025 Jul 24;10(30):33946-33958. doi: 10.1021/acsomega.5c06385. eCollection 2025 Aug 5. ACS Omega. 2025. PMID: 40787353 Free PMC article.
-
Design and optimization of PLGA-based gemcitabine nanocapsule for enhanced pancreatic cancer efficacy.Invest New Drugs. 2025 Jul 23. doi: 10.1007/s10637-025-01567-y. Online ahead of print. Invest New Drugs. 2025. PMID: 40696247
-
Biodegradable Polymersomes for the Delivery of Gemcitabine to Panc-1 Cells.J Pharm (Cairo). 2013;2013:932797. doi: 10.1155/2013/932797. J Pharm (Cairo). 2013. PMID: 26167335 Free PMC article.
References
-
- Kamath A, Yoo D, Stuart OA, Bijelic L, Sugarbaker PH. Rationale for an intraperitoneal gemcitabine chemotherapy treatment for patients with resected pancreatic cancer. Recent Pat Anticancer Drug Discov. 2009;4:174–179. - PubMed
-
- Danesi R, Altavilla G, Giovannetti E, Rosell R. Pharmacogenomics of gemcitabine in non-small-cell lung cancer and other solid tumors. Pharmacogenomics. 2009;10:69–80. - PubMed
-
- Reddy LH, Dubernet C, Mouelhi SL, Marque PE, Desmaele D, Couvreur P. A new nanomedicine of gemcitabine displays enhanced anticancer activity in sensitive and resistant leukemia types. J Control Release. 2007;124:20–27. - PubMed
-
- Chen C, Lv G, Pan C, Song M, Wu C, Guo D, Wang X, Chen B, Gu Z. Poly(lactic acid) (PLA) based nanocomposites--a novel way of drug-releasing. Biomed Mater. 2007;2:L1–L4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

