Bioconjugation of calcium phosphosilicate composite nanoparticles for selective targeting of human breast and pancreatic cancers in vivo
- PMID: 20180585
- PMCID: PMC2894697
- DOI: 10.1021/nn901297q
Bioconjugation of calcium phosphosilicate composite nanoparticles for selective targeting of human breast and pancreatic cancers in vivo
Abstract
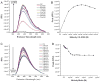
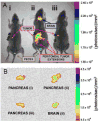
The early diagnosis of cancer is the critical element in successful treatment and long-term favorable patient prognoses. The high rate of mortality is mainly attributed to the tendency for late diagnoses as symptoms may not occur until the disease has metastasized, as well as the lack of effective systemic therapies. Late diagnosis is often associated with the lack of timely sensitive imaging modalities. The promise of nanotechnology is presently limited by the inability to simultaneously seek, treat, and image cancerous lesions. This study describes the design and synthesis of fluorescent calcium phosphosilicate nanocomposite particles (CPNPs) that can be systemically targeted to breast and pancreatic cancer lesions. The CPNPs are a approximately 20 nm diameter composite composed of an amorphous calcium phosphate matrix doped with silicate in which a near-infrared imaging agent, indocyanine green (ICG), is embedded. In the present studies, we describe and validate CPNP bioconjugation of human holotransferrin, anti-CD71 antibody, and short gastrin peptides via an avidin-biotin or a novel PEG-maleimide coupling strategy. The conjugation of biotinylated human holotransferrin (diferric transferrin) and biotinylated anti-CD71 antibody (anti-transferrin receptor antibody) to avidin-conjugated CPNPs (Avidin-CPNPs) permits targeting of transferrin receptors, which are highly expressed on breast cancer cells. Similarly, the conjugation of biotinylated pentagastrin to Avidin-CPNPs and decagastrin (gastrin-10) to PEG-CPNPs via PEG-maleimide coupling permits targeting of gastrin receptors, which are overexpressed in pancreatic cancer lesions. These bioconjugated CPNPs have the potential to perform as a theranostic modality, simultaneously enhancing drug delivery, targeting, and imaging of breast and pancreatic cancer tumors.
Figures






Similar articles
-
Near-infrared emitting fluorophore-doped calcium phosphate nanoparticles for in vivo imaging of human breast cancer.ACS Nano. 2008 Oct 28;2(10):2075-84. doi: 10.1021/nn800448r. ACS Nano. 2008. PMID: 19206454
-
Aptamer-Targeted Calcium Phosphosilicate Nanoparticles for Effective Imaging of Pancreatic and Prostate Cancer.Int J Nanomedicine. 2021 Mar 19;16:2297-2309. doi: 10.2147/IJN.S295740. eCollection 2021. Int J Nanomedicine. 2021. PMID: 33776434 Free PMC article.
-
Targeted indocyanine-green-loaded calcium phosphosilicate nanoparticles for in vivo photodynamic therapy of leukemia.ACS Nano. 2011 Jul 26;5(7):5325-37. doi: 10.1021/nn2005766. Epub 2011 Jul 8. ACS Nano. 2011. PMID: 21675727
-
What are the pancreatic target cells for gastrin and its CCKB receptor? Is this a couple for cancerous cells?Med Sci Monit. 2004 Oct;10(10):RA242-6. Epub 2004 Sep 23. Med Sci Monit. 2004. PMID: 15448615 Review.
-
Polyethylene glycol–coated and folic acid–conjugated superparamagnetic iron oxide nanoparticles.2009 Sep 30 [updated 2009 Nov 12]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2009 Sep 30 [updated 2009 Nov 12]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641868 Free Books & Documents. Review.
Cited by
-
A review of indocyanine green fluorescent imaging in surgery.Int J Biomed Imaging. 2012;2012:940585. doi: 10.1155/2012/940585. Epub 2012 Apr 22. Int J Biomed Imaging. 2012. PMID: 22577366 Free PMC article.
-
Preferential uptake of antibody targeted calcium phosphosilicate nanoparticles by metastatic triple negative breast cancer cells in co-cultures of human metastatic breast cancer cells plus bone osteoblasts.Nanomedicine. 2021 Jun;34:102383. doi: 10.1016/j.nano.2021.102383. Epub 2021 Mar 13. Nanomedicine. 2021. PMID: 33722692 Free PMC article.
-
Nanotechnology for Topical Drug Delivery to the Anterior Segment of the Eye.Int J Mol Sci. 2021 Nov 16;22(22):12368. doi: 10.3390/ijms222212368. Int J Mol Sci. 2021. PMID: 34830247 Free PMC article. Review.
-
Hand-held spectroscopic device for in vivo and intraoperative tumor detection: contrast enhancement, detection sensitivity, and tissue penetration.Anal Chem. 2010 Nov 1;82(21):9058-65. doi: 10.1021/ac102058k. Epub 2010 Oct 6. Anal Chem. 2010. PMID: 20925393 Free PMC article.
-
Responsive theranostic systems: integration of diagnostic imaging agents and responsive controlled release drug delivery carriers.Acc Chem Res. 2011 Oct 18;44(10):1061-70. doi: 10.1021/ar2001777. Epub 2011 Sep 20. Acc Chem Res. 2011. PMID: 21932809 Free PMC article. Review.
References
-
- Morgan TT, Muddana HS, Altınoğlu Eİ, Rouse SM, Tabaković A, Tabouillot T, Russin TJ, Shanmugavelandy SS, Butler PJ, Eklund PC, et al. Encapsulation of Organic Molecules in Calcium Phosphate Nanocomposite Particles for Intracellular Imaging and Drug Delivery. Nano Letters. 2008;8(12):4108–4115. - PMC - PubMed
-
- Altınoğlu Eİ, Russin TJ, Kaiser JM, Barth BM, Eklund PC, Kester M, Adair JH. Near-Infrared Emitting Fluorophore-Doped Calcium Phosphate Nanoparticles for in Vivo Imaging of Human Breast Cancer. ACS Nano. 2008;2(10):2075–2084. - PubMed
-
- Daniels TR, Delgado T, Helguera G, Penichet ML. The Transferrin Receptor Part Ii: Targeted Delivery of Therapeutic Agents into Cancer Cells. Clin Immunol. 2006;121(2):159–176. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

