Frequent transcriptional inactivation of Kallikrein 10 gene by CpG island hypermethylation in non-small cell lung cancer
- PMID: 20180809
- PMCID: PMC11158746
- DOI: 10.1111/j.1349-7006.2009.01486.x
Frequent transcriptional inactivation of Kallikrein 10 gene by CpG island hypermethylation in non-small cell lung cancer
Abstract
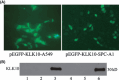
The role of Kallikrein 10 gene (KLK10) in non-small cell lung cancer (NSCLC) remains largely unknown. We determined the frequency and functional significance of KLK10 hypermethylation in NSCLC. The mRNA expression and methylation status of KLK10 in 78 pairs NSCLC specimens was explored. The biological effects of KLK10 were analyzed by transfection. The results showed that, KLK10 was significantly downregulated in NSCLC (57.7%, 45/78) as compared to non-cancer samples (P = 0.010). CpG island hypermethylation of KLK10 was detected in 46.2% (36/78) NSCLC tissues and was closely correlated with loss of transcript (P < 0.001). KLK10 methylation was associated with advanced stage (P = 0.013) and lymph metastasis (P = 0.015). Furthermore, demethylation treatment restored the expression of KLK10 in two lung adencarcinoma cell lines (A549, SPC-A1). Forced expression of KLK10 in A549 and SPC-A1 remarkably suppressed cells proliferation, migration in vitro and oncogenicity in vivo. Additionally, methylated KLK10 was detected in 38.7% (30/78) of plasma samples from cancer patients but rare in cancer-free controls (P < 0.001). In conclusion, KLK10 acts as a functional tumor suppressor gene in NSCLC, epigenetic inactivation of KLK10 is a common event contributing to NSCLC pathogenesis and may be used as a potential biomarker.
Figures






Similar articles
-
Downregulation of human kallikrein 10 (KLK10/NES1) by CpG island hypermethylation in breast, ovarian and prostate cancers.Tumour Biol. 2005 Nov-Dec;26(6):324-36. doi: 10.1159/000089290. Epub 2005 Oct 26. Tumour Biol. 2005. PMID: 16254462
-
Methylation of multiple genes as a candidate biomarker in non-small cell lung cancer.Cancer Lett. 2011 Apr 1;303(1):21-8. doi: 10.1016/j.canlet.2010.12.011. Epub 2011 Jan 20. Cancer Lett. 2011. PMID: 21255913
-
Frequent epigenetic inactivation of deleted in lung and esophageal cancer 1 gene by promoter methylation in non-small-cell lung cancer.Clin Lung Cancer. 2010 Jul 1;11(4):264-70. doi: 10.3816/CLC.2010.n.034. Clin Lung Cancer. 2010. PMID: 20630829
-
5' CpG island methylation of the FHIT gene is correlated with loss of gene expression in lung and breast cancer.Cancer Res. 2001 May 1;61(9):3581-5. Cancer Res. 2001. PMID: 11325823
-
Aberrant DNA methylation profile and frequent methylation of KLK10 and OXGR1 genes in hepatocellular carcinoma.Genes Chromosomes Cancer. 2009 Dec;48(12):1057-68. doi: 10.1002/gcc.20708. Genes Chromosomes Cancer. 2009. PMID: 19760608
Cited by
-
MicroRNA-375 Suppresses Extracellular Matrix Degradation and Invadopodial Activity in Head and Neck Squamous Cell Carcinoma.Arch Pathol Lab Med. 2015 Nov;139(11):1349-61. doi: 10.5858/arpa.2014-0471-OA. Epub 2015 Jul 14. Arch Pathol Lab Med. 2015. PMID: 26172508 Free PMC article.
-
Methylated Circulating Tumor DNA in Blood as a Tool for Diagnosing Lung Cancer: A Systematic Review and Meta-Analysis.Cancers (Basel). 2023 Aug 3;15(15):3959. doi: 10.3390/cancers15153959. Cancers (Basel). 2023. PMID: 37568774 Free PMC article. Review.
-
Kallikrein-related peptidase 13: an independent indicator of favorable prognosis for patients with nonsmall cell lung cancer.Tumour Biol. 2015 Jul;36(7):4979-86. doi: 10.1007/s13277-015-3148-1. Epub 2015 Feb 13. Tumour Biol. 2015. PMID: 25677900
-
Deep-LC: A Novel Deep Learning Method of Identifying Non-Small Cell Lung Cancer-Related Genes.Front Oncol. 2022 Jul 22;12:949546. doi: 10.3389/fonc.2022.949546. eCollection 2022. Front Oncol. 2022. PMID: 35936745 Free PMC article.
-
DNA Methylation-Based Testing in Liquid Biopsies as Detection and Prognostic Biomarkers for the Four Major Cancer Types.Cells. 2020 Mar 5;9(3):624. doi: 10.3390/cells9030624. Cells. 2020. PMID: 32150897 Free PMC article. Review.
References
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics 2009. CA Cancer J Clin 2009; 59: 225–49. - PubMed
-
- Minna JD, Roth JA, Gazdar AF. Focus on lung cancer. Cancer Cell 2002; 1: 49–52. - PubMed
-
- Baylin SB, Herman JG. DNA hypermethylation in tumorigenesis: epigenetics joins genetics. Trends Genet 2000; 16: 168–74. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

