Improving calculation, interpretation and communication of familial colorectal cancer risk: protocol for a randomized controlled trial
- PMID: 20181032
- PMCID: PMC2832626
- DOI: 10.1186/1748-5908-5-6
Improving calculation, interpretation and communication of familial colorectal cancer risk: protocol for a randomized controlled trial
Abstract
Background: Individuals with multiple relatives with colorectal cancer (CRC) and/or a relative with early-onset CRC have an increased risk of developing CRC. They are eligible for preventive measures, such as surveillance by regular colonoscopy and/or genetic counselling. Currently, most at-risk individuals do not follow the indicated follow-up policy. In a new guideline on familial and hereditary CRC, clinicians have new tasks in calculating, interpreting, and communicating familial CRC risk. This will lead to better recognition of individuals at an increased familial CRC risk, enabling them to take effective preventive measures. This trial compares two implementation strategies (a common versus an intensive implementation strategy), focussing on clinicians' risk calculation, interpretation, and communication, as well as patients' uptake of the indicated follow-up policy.
Methods: A clustered randomized controlled trial including an effect, process, and cost evaluation will be conducted in eighteen hospitals. Nine hospitals in the control group will receive the common implementation strategy (i.e., dissemination of the guideline). In the intervention group, an intensive implementation strategy will be introduced. Clinicians will receive education and tools for risk calculation, interpretation, and communication. Patients will also receive these tools, in addition to patient decision aids. The effect evaluation includes assessment of the number of patients for whom risk calculation, interpretation, and communication is performed correctly, and the number of patients following the indicated follow-up policy. The actual exposure to the implementation strategies and users' experiences will be assessed in the process evaluation. In a cost evaluation, the costs of the implementation strategies will be determined.
Discussion: The results of this study will help determine the most effective method as well as the costs of improving the recognition of individuals at an increased familial CRC risk. It will provide insight into the experiences of both patients and clinicians with these strategies.The knowledge gathered in this study can be used to improve the recognition of familial and hereditary CRC at both the national and international level, and will serve as an example to improve care for patients and their relatives worldwide. Our results may also be useful in improving healthcare in other diseases.
Trial registration: ClinicalTrials.gov NCT00929097.
Figures
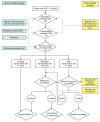
References
-
- The Netherlands Cancer Registry and Statistics 1999-2003. Netherlands. 2003.
-
- de Jong AE, Vasen HF. The frequency of a positive family history for colorectal cancer: a population-based study in the Netherlands. Neth J Med. 2006;64:367–370. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

