Expression of interleukin-1 (IL-1) ligands system in the most common endometriosis-associated ovarian cancer subtypes
- PMID: 20181040
- PMCID: PMC2832771
- DOI: 10.1186/1757-2215-3-3
Expression of interleukin-1 (IL-1) ligands system in the most common endometriosis-associated ovarian cancer subtypes
Abstract
Objectives: Endometrioid carcinoma of the ovary is one of the most types of epithelial ovarian cancer associated to endometrioisis. Endometrioid tumors as well as endometriotic implants are characterized by the presence of epithelial cells, stromal cells, or a combination of booth, that resemble the endometrial cells, suggesting a possible endometrial origin of these tumors. Pro-inflammatory cytokines, including interleukin-1 (IL-1) have been reported to be involved in both endometriosis and ovarian carcinogenesis. The major objective of this study was to determine the level expression of IL-1 ligands system (IL-1alpha, IL-1beta and IL-1RA) in the most common subtypes of ovarian cancer cells compared to endometrial cells.
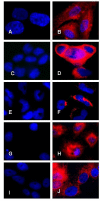
Methods: We used primary endometrial cells, endometrial cell line RL-952 and different subtypes of epithelial ovarian cancer cell lines including TOV-112D (endometrioid), TOV-21G (clear cell) and OV-90 (serous). Immunofluorescence and real-time PCR analysis were used respectively for detecting IL-1 ligands at the levels of cell-associated protein and mRNA. Soluble IL-1 ligands were analyzed by ELISA.
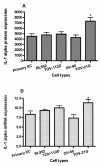
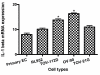
Results: We demonstrated that IL-1 ligands were expressed by all endometriosis-associated ovarian cancer subtypes and endometrial cells. In contrast to other cancer ovarian cells, endometrioid cells exhibit a specific decrease of cell-associated IL-1RA expression and its soluble secretion.
Conclusion: Endometrioid ovarian cancer exhibits an alteration in the expression of IL-1RA, a key protector against tumorogenic effects of IL-1. This alteration evokes the same alteration observed in endometriotic cells in previous studies. This suggests a possible link between the endometrium, the tissue ectopic endometriosis and endometrioid ovarian cancer.
Figures


Similar articles
-
Endometrioid ovarian cancer and endometriotic cells exhibit the same alteration in the expression of interleukin-1 receptor II: to a link between endometriosis and endometrioid ovarian cancer.J Obstet Gynaecol Res. 2011 Feb;37(2):99-107. doi: 10.1111/j.1447-0756.2010.01320.x. Epub 2010 Nov 18. J Obstet Gynaecol Res. 2011. PMID: 21083841
-
Gene expression profiles of serous, endometrioid, and clear cell subtypes of ovarian and endometrial cancer.Clin Cancer Res. 2005 Sep 15;11(18):6422-30. doi: 10.1158/1078-0432.CCR-05-0508. Clin Cancer Res. 2005. PMID: 16166416
-
Frequent PTEN/MMAC mutations in endometrioid but not serous or mucinous epithelial ovarian tumors.Cancer Res. 1998 May 15;58(10):2095-7. Cancer Res. 1998. PMID: 9605750
-
Association between endometriosis and gynecological cancers: a critical review of the literature.Arch Gynecol Obstet. 2020 Feb;301(2):355-367. doi: 10.1007/s00404-020-05445-1. Epub 2020 Feb 5. Arch Gynecol Obstet. 2020. PMID: 32025845 Review.
-
Comparison of clinical outcomes of patients with clear cell and endometrioid ovarian cancer associated with endometriosis to papillary serous carcinoma of the ovary.Gynecol Oncol. 2014 Mar;132(3):760-6. doi: 10.1016/j.ygyno.2014.01.012. Epub 2014 Jan 16. Gynecol Oncol. 2014. PMID: 24440832 Review.
Cited by
-
Plasma glutamate-modulated interaction of A2AR and mGluR5 on BMDCs aggravates traumatic brain injury-induced acute lung injury.J Exp Med. 2013 Apr 8;210(4):839-51. doi: 10.1084/jem.20122196. Epub 2013 Mar 11. J Exp Med. 2013. PMID: 23478188 Free PMC article. Clinical Trial.
-
Down Expression of Zyxin is Associated with Down Expression of p53 in Colorectal Cancer.Int J Mol Cell Med. 2025;14(1):461-471. doi: 10.22088/IJMCM.BUMS.14.1.462. Int J Mol Cell Med. 2025. PMID: 40123589 Free PMC article.
-
The Relationship Between Cytokine Production, CSF2RA, and IL1R2 Expression in Mammary Adenocarcinoma, Tumor Histopathological Parameters, and Lymph Node Metastasis.Technol Cancer Res Treat. 2019 Jan-Dec;18:1533033819883626. doi: 10.1177/1533033819883626. Technol Cancer Res Treat. 2019. PMID: 31635541 Free PMC article.
-
NOD-like receptors: major players (and targets) in the interface between innate immunity and cancer.Biosci Rep. 2019 Apr 9;39(4):BSR20181709. doi: 10.1042/BSR20181709. Print 2019 Apr 30. Biosci Rep. 2019. PMID: 30837326 Free PMC article. Review.
-
The Expression of TGF-β1, SMAD3, ILK and miRNA-21 in the Ectopic and Eutopic Endometrium of Women with Endometriosis.Int J Mol Sci. 2023 Jan 26;24(3):2453. doi: 10.3390/ijms24032453. Int J Mol Sci. 2023. PMID: 36768775 Free PMC article.
References
-
- Somigliana E, Vigano P, Parazzini F, Stoppelli S, Giambattista E, Vercellini P. Association between endometriosis and cancer: a comprehensive review and a critical analysis of clinical and epidemiological evidence. Gynecologic oncology. 2006;101:331–341. doi: 10.1016/j.ygyno.2005.11.033. - DOI - PubMed
LinkOut - more resources
Full Text Sources

