Self-organization of intracellular gradients during mitosis
- PMID: 20181052
- PMCID: PMC2829544
- DOI: 10.1186/1747-1028-5-5
Self-organization of intracellular gradients during mitosis
Abstract
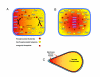
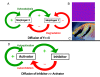
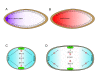
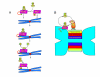
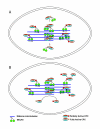
Gradients are used in a number of biological systems to transmit spatial information over a range of distances. The best studied are morphogen gradients where information is transmitted over many cell lengths. Smaller mitotic gradients reflect the need to organize several distinct events along the length of the mitotic spindle. The intracellular gradients that characterize mitosis are emerging as important regulatory paradigms. Intracellular gradients utilize intrinsic auto-regulatory feedback loops and diffusion to establish stable regions of activity within the mitotic cytosol. We review three recently described intracellular mitotic gradients. The Ran GTP gradient with its elaborate cascade of nuclear transport receptors and cargoes is the best characterized, yet the dynamics underlying the robust gradient of Ran-GTP have received little attention. Gradients of phosphorylation have been observed on Aurora B kinase substrates both before and after anaphase onset. In both instances the phosphorylation gradient appears to result from a soluble gradient of Aurora B kinase activity. Regulatory properties that support gradient formation are highlighted. Intracellular activity gradients that regulate localized mitotic events bare several hallmarks of self-organizing biologic systems that designate spatial information during pattern formation. Intracellular pattern formation represents a new paradigm in mitotic regulation.
Figures


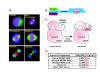
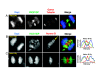
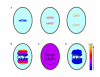
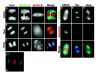
References
LinkOut - more resources
Full Text Sources
Miscellaneous

