Differential impact of ageing on cellular and humoral immunity to a persistent murine gamma-herpesvirus
- PMID: 20181071
- PMCID: PMC2843645
- DOI: 10.1186/1742-4933-7-3
Differential impact of ageing on cellular and humoral immunity to a persistent murine gamma-herpesvirus
Abstract
Background: Oncogenic gamma-herpesviruses establish life-long infections in their hosts and control of these latent infections is dependent on continual immune surveillance. Immune function declines with age, raising the possibility that immune control of gamma-herpesvirus infection becomes compromised with increasing age, allowing viral reactivation and/or increased latent load, both of which are associated with the development of malignancies.
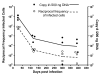
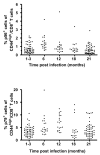
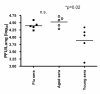
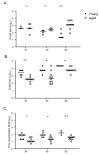
Results: In this study, we use the experimental mouse gamma-herpesvirus model, gammaHV68, to investigate viral immunity in aged mice. We found no evidence of viral recrudescence or increased latent load in aged latently-infected mice, suggesting that effective immune control of gamma-herpesvirus infection remains intact with ageing. As both cellular and humoral immunity have been implicated in host control of gammaHV68 latency, we independently examined the impact of ageing on gammaHV68-specific CD8 T cell function and antibody responses. Virus-specific CD8 T cell numbers and cytolytic function were not profoundly diminished with age. In contrast, whereas ELISA titers of virus-specific IgG were maintained over time, there was a progressive decline in neutralizing activity. In addition, although aged mice were able to control de novo acute infection with only slightly delayed viral clearance, serum titers of neutralizing antibody were reduced in aged mice as compared to young mice.
Conclusion: Although there is no obvious loss of immune control of latent virus, these data indicate that ageing has differential impacts on anti-viral cellular and humoral immune protection during persistent gammaHV68 infection. This observation has potential relevance for understanding gamma-herpesvirus immune control during disease-associated or therapeutic immunosuppression.
Figures

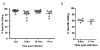



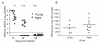

Similar articles
-
Importance of antibody in virus infection and vaccine-mediated protection by a latency-deficient recombinant murine γ-herpesvirus-68.J Immunol. 2012 Feb 1;188(3):1049-56. doi: 10.4049/jimmunol.1102621. Epub 2011 Dec 23. J Immunol. 2012. PMID: 22198955 Free PMC article.
-
A replication-deficient murine gamma-herpesvirus blocked in late viral gene expression can establish latency and elicit protective cellular immunity.J Immunol. 2007 Dec 15;179(12):8392-402. doi: 10.4049/jimmunol.179.12.8392. J Immunol. 2007. PMID: 18056385
-
Recombinant Murine Gamma Herpesvirus 68 Carrying KSHV G Protein-Coupled Receptor Induces Angiogenic Lesions in Mice.PLoS Pathog. 2015 Jun 24;11(6):e1005001. doi: 10.1371/journal.ppat.1005001. eCollection 2015 Jun. PLoS Pathog. 2015. PMID: 26107716 Free PMC article.
-
Unraveling immunity to gamma-herpesviruses: a new model for understanding the role of immunity in chronic virus infection.Curr Opin Immunol. 1999 Aug;11(4):371-9. doi: 10.1016/s0952-7915(99)80063-6. Curr Opin Immunol. 1999. PMID: 10448140 Review.
-
Natural history of murine gamma-herpesvirus infection.Philos Trans R Soc Lond B Biol Sci. 2001 Apr 29;356(1408):569-79. doi: 10.1098/rstb.2000.0779. Philos Trans R Soc Lond B Biol Sci. 2001. PMID: 11313012 Free PMC article. Review.
Cited by
-
Viral infection and aging as cofactors for the development of pulmonary fibrosis.Expert Rev Respir Med. 2010 Dec;4(6):759-71. doi: 10.1586/ers.10.73. Expert Rev Respir Med. 2010. PMID: 21128751 Free PMC article. Review.
-
Immune aging and challenges for immune protection of the graying population.Aging Dis. 2011 Oct;2(5):339-45. Epub 2011 Oct 28. Aging Dis. 2011. PMID: 22396886 Free PMC article. No abstract available.
-
Mechanisms of immunosenescence: lessons from models of accelerated immune aging.Ann N Y Acad Sci. 2012 Jan;1247:69-82. doi: 10.1111/j.1749-6632.2011.06297.x. Epub 2012 Jan 6. Ann N Y Acad Sci. 2012. PMID: 22224726 Free PMC article.
-
Importance of antibody in virus infection and vaccine-mediated protection by a latency-deficient recombinant murine γ-herpesvirus-68.J Immunol. 2012 Feb 1;188(3):1049-56. doi: 10.4049/jimmunol.1102621. Epub 2011 Dec 23. J Immunol. 2012. PMID: 22198955 Free PMC article.
-
Pulmonary fibrosis induced by γ-herpesvirus in aged mice is associated with increased fibroblast responsiveness to transforming growth factor-β.J Gerontol A Biol Sci Med Sci. 2012 Jun;67(7):714-25. doi: 10.1093/gerona/glr211. Epub 2011 Dec 21. J Gerontol A Biol Sci Med Sci. 2012. PMID: 22193547 Free PMC article.
References
-
- Pawelec G, Larbi A. Immunity and ageing in man: Annual Review 2006/2007. Exp Gerontol. 2008;43(1):34–38. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

