Roles for common MLL/COMPASS subunits and the 19S proteasome in regulating CIITA pIV and MHC class II gene expression and promoter methylation
- PMID: 20181089
- PMCID: PMC2829561
- DOI: 10.1186/1756-8935-3-5
Roles for common MLL/COMPASS subunits and the 19S proteasome in regulating CIITA pIV and MHC class II gene expression and promoter methylation
Abstract
Background: Studies indicate that the 19S proteasome contributes to chromatin reorganization, independent of the role the proteasome plays in protein degradation. We have previously shown that components of the 19S proteasome are crucial for regulating inducible histone activation events in mammalian cells. The 19S ATPase Sug1 binds to histone-remodeling enzymes, and in the absence of Sug1, a subset of activating epigenetic modifications including histone H3 acetylation, H3 lysine 4 trimethylation and H3 arginine 17 dimethylation are inhibited at cytokine-inducible major histocompatibilty complex (MHC)-II and class II transactivator (CIITA) promoters, implicating Sug1 in events required to initiate mammalian transcription.
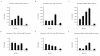
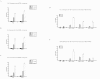
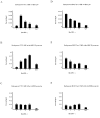
Results: Our previous studies indicate that H3 lysine 4 trimethylation at cytokine-inducible MHC-II and CIITA promoters is dependent on proteolytic-independent functions of 19S ATPases. In this report, we show that multiple common subunits of the mixed lineage leukemia (MLL)/complex of proteins associated with Set I (COMPASS) complexes bind to the inducible MHC-II and CIITA promoters; that overexpressing a single common MLL/COMPASS subunit significantly enhances promoter activity and MHC-II HLA-DRA expression; and that these common subunits are important for H3 lysine 4 trimethylation at MHC-II and CIITA promoters. In addition, we show that H3 lysine 27 trimethylation, which is inversely correlated with H3 lysine 4 trimethylation, is significantly elevated in the presence of diminished 19S ATPase Sug1.
Conclusion: Taken together, these experiments suggest that the 19S proteasome plays a crucial role in the initial reorganization of events enabling the relaxation of the repressive chromatin structure surrounding inducible promoters.
Figures
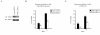

References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

