Histone deacetylase inhibitors induce apoptosis in human eosinophils and neutrophils
- PMID: 20181093
- PMCID: PMC2841159
- DOI: 10.1186/1476-9255-7-9
Histone deacetylase inhibitors induce apoptosis in human eosinophils and neutrophils
Abstract
Background: Granulocytes are important in the pathogenesis of several inflammatory diseases. Apoptosis is pivotal in the resolution of inflammation. Apoptosis in malignant cells is induced by histone deacetylase (HDAC) inhibitors, whereas HDAC inhibitors do not usually induce apoptosis in non-malignant cells. The aim of the present study was to explore the effects of HDAC inhibitors on apoptosis in human eosinophils and neutrophils.
Methods: Apoptosis was assessed by relative DNA fragmentation assay, annexin-V binding, and morphologic analysis. HDAC activity in nuclear extracts was measured with a nonisotopic assay. HDAC expression was measured by real-time PCR.
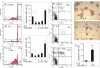
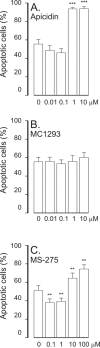
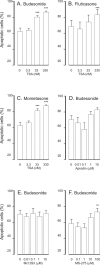
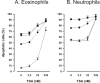
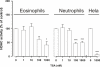
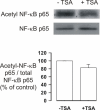
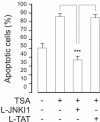
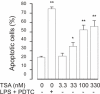
Results: A HDAC inhibitor Trichostatin A (TSA) induced apoptosis in the presence of survival-prolonging cytokines interleukin-5 and granulocyte-macrophage colony stimulating factor (GM-CSF) in eosinophils and neutrophils. TSA enhanced constitutive eosinophil and neutrophil apoptosis. Similar effects were seen with a structurally dissimilar HDAC inhibitor apicidin. TSA showed additive effect on the glucocorticoid-induced eosinophil apoptosis, but antagonized glucocorticoid-induced neutrophil survival. Eosinophils and neutrophils expressed all HDACs at the mRNA level except that HDAC5 and HDAC11 mRNA expression was very low in both cell types, HDAC8 mRNA was very low in neutrophils and HDAC9 mRNA low in eosinophils. TSA reduced eosinophil and neutrophil nuclear HDAC activities by ~50-60%, suggesting a non-histone target. However, TSA did not increase the acetylation of a non-histone target NF-kappaB p65. c-jun-N-terminal kinase and caspases 3 and 6 may be involved in the mechanism of TSA-induced apoptosis, whereas PI3-kinase and caspase 8 are not.
Conclusions: HDAC inhibitors enhance apoptosis in human eosinophils and neutrophils in the absence and presence of survival-prolonging cytokines and glucocorticoids.
Figures
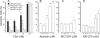
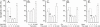


References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

