Optimised electroporation mediated DNA vaccination for treatment of prostate cancer
- PMID: 20181099
- PMCID: PMC2829554
- DOI: 10.1186/1479-0556-8-1
Optimised electroporation mediated DNA vaccination for treatment of prostate cancer
Abstract
Background: Immunological therapies enhance the ability of the immune system to recognise and destroy cancer cells via selective killing mechanisms. DNA vaccines have potential to activate the immune system against specific antigens, with accompanying potent immunological adjuvant effects from unmethylated CpG motifs as on prokaryotic DNA. We investigated an electroporation driven plasmid DNA vaccination strategy in animal models for treatment of prostate cancer.
Methods: Plasmid expressing human PSA gene (phPSA) was delivered in vivo by intra-muscular electroporation, to induce effective anti-tumour immune responses against prostate antigen expressing tumours. Groups of male C57 BL/6 mice received intra-muscular injections of phPSA plasmid. For phPSA delivery, quadriceps muscle was injected with 50 microg plasmid. After 80 seconds, square-wave pulses were administered in sequence using a custom designed pulse generator and a custom-designed applicator with 2 needles placed through the skin central to the muscle. To determine an optimum treatment regimen, three different vaccination schedules were investigated. In a separate experiment, the immune potential of the phPSA vaccine was further enhanced with co- administration of synthetic CpG rich oligonucleotides. One week after last vaccination, the mice were challenged subcutaneously with TRAMPC1/hPSA (prostate cancer cell line stably expressing human PSA) and tumour growth was monitored. Serum from animals was examined by ELISA for anti-hPSA antibodies and for IFN gamma. Histological assessment of the tumours was also carried out. In vivo and in vitro cytotoxicity assays were performed with splenocytes from treated mice.
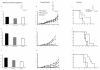
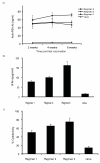
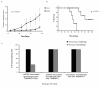
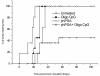
Results: The phPSA vaccine therapy significantly delayed the appearance of tumours and resulted in prolonged survival of the animals. Four-dose vaccination regimen provided optimal immunological effects. Co - administration of the synthetic CpG with phPSA increased anti-tumour responses, preventing tumour occurrence in 54% of treated animals. Vaccination with phPSA resulted in anti-hPSA Abs production and a significant production of IFN gamma was observed in immunised animals (p < 0.05). Immune responses were tumour specific and were transferable in adoptive T cell transfer experiments.
Conclusions: This phPSA plasmid electroporation vaccination strategy can effectively activate tumour specific immune responses. Optimisation of the approach indicated that a four-dose regimen provided highest tumour protection. In vivo electroporation mediated vaccination is a safe and effective modality for the treatment of prostate cancer and has a potential to be used as a neo-adjuvant or adjuvant therapy.
Figures


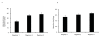
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

