Phosphoproteomes of Strongylocentrotus purpuratus shell and tooth matrix: identification of a major acidic sea urchin tooth phosphoprotein, phosphodontin
- PMID: 20181113
- PMCID: PMC2830187
- DOI: 10.1186/1477-5956-8-6
Phosphoproteomes of Strongylocentrotus purpuratus shell and tooth matrix: identification of a major acidic sea urchin tooth phosphoprotein, phosphodontin
Abstract
Background: Sea urchin is a major model organism for developmental biology and biomineralization research. However, identification of proteins involved in larval skeleton formation and mineralization processes in the embryo and adult, and the molecular characterization of such proteins, has just gained momentum with the sequencing of the Strongylocentrotus purpuratus genome and the introduction of high-throughput proteomics into the field.
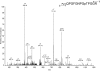
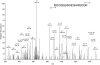
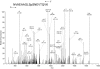
Results: The present report contains the determination of test (shell) and tooth organic matrix phosphoproteomes. Altogether 34 phosphoproteins were identified in the biomineral organic matrices. Most phosphoproteins were specific for one compartment, only two were identified in both matrices. The sea urchin phosphoproteomes contained several obvious orthologs of mammalian proteins, such as a Src family tyrosine kinase, protein kinase C-delta 1, Dickkopf-1 and other signal transduction components, or nucleobindin. In most cases phosphorylation sites were conserved between sea urchin and mammalian proteins. However, the majority of phosphoproteins had no mammalian counterpart. The most interesting of the sea urchin-specific phosphoproteins, from the perspective of biomineralization research, was an abundant highly phosphorylated and very acidic tooth matrix protein composed of 35 very similar short sequence repeats, a predicted N-terminal secretion signal sequence, and an Asp-rich C-terminal motif, contained in [Glean3:18919].
Conclusions: The 64 phosphorylation sites determined represent the most comprehensive list of experimentally identified sea urchin protein phosphorylation sites at present and are an important addition to the recently analyzed Strongylocentrotus purpuratus shell and tooth proteomes. The identified phosphoproteins included a major, highly phosphorylated protein, [Glean3:18919], for which we suggest the name phosphodontin. Although not sequence-related to such highly phosphorylated acidic mammalian dental phosphoproteins as phosphoryn or dentin matrix protein-1, phosphodontin may perform similar functions in the sea urchin tooth. More than half of the detected proteins were not previously identified at the protein level, thus confirming the existence of proteins only known as genomic sequences previously.
Figures


Similar articles
-
The sea urchin (Strongylocentrotus purpuratus) test and spine proteomes.Proteome Sci. 2008 Aug 11;6:22. doi: 10.1186/1477-5956-6-22. Proteome Sci. 2008. PMID: 18694502 Free PMC article.
-
In-depth, high-accuracy proteomics of sea urchin tooth organic matrix.Proteome Sci. 2008 Dec 9;6:33. doi: 10.1186/1477-5956-6-33. Proteome Sci. 2008. PMID: 19068105 Free PMC article.
-
Proteomic analysis of sea urchin (Strongylocentrotus purpuratus) spicule matrix.Proteome Sci. 2010 Jun 17;8:33. doi: 10.1186/1477-5956-8-33. Proteome Sci. 2010. PMID: 20565753 Free PMC article.
-
Identification and developmental expression of the ets gene family in the sea urchin (Strongylocentrotus purpuratus).Dev Biol. 2006 Dec 1;300(1):35-48. doi: 10.1016/j.ydbio.2006.08.012. Epub 2006 Aug 10. Dev Biol. 2006. PMID: 16997294 Review.
-
Ocean acidification research in the 'post-genomic' era: Roadmaps from the purple sea urchin Strongylocentrotus purpuratus.Comp Biochem Physiol A Mol Integr Physiol. 2015 Jul;185:33-42. doi: 10.1016/j.cbpa.2015.03.007. Epub 2015 Mar 13. Comp Biochem Physiol A Mol Integr Physiol. 2015. PMID: 25773301 Review.
Cited by
-
Molecular cloning and characterization of first organic matrix protein from sclerites of red coral, Corallium rubrum.J Biol Chem. 2012 Jun 1;287(23):19367-76. doi: 10.1074/jbc.M112.352005. Epub 2012 Apr 13. J Biol Chem. 2012. PMID: 22505718 Free PMC article.
-
The isolation and characterization of glycosylated phosphoproteins from herring fish bones.J Biol Chem. 2010 Nov 12;285(46):36170-8. doi: 10.1074/jbc.M110.146910. Epub 2010 Sep 10. J Biol Chem. 2010. PMID: 20833721 Free PMC article.
-
The Evolution of Biomineralization through the Co-Option of Organic Scaffold Forming Networks.Cells. 2022 Feb 9;11(4):595. doi: 10.3390/cells11040595. Cells. 2022. PMID: 35203246 Free PMC article. Review.
-
Organic matrix-related mineralization of sea urchin spicules, spines, test and teeth.Front Biosci (Landmark Ed). 2011 Jun 1;16(7):2540-60. doi: 10.2741/3871. Front Biosci (Landmark Ed). 2011. PMID: 21622194 Free PMC article. Review.
-
The Lottia gigantea shell matrix proteome: re-analysis including MaxQuant iBAQ quantitation and phosphoproteome analysis.Proteome Sci. 2014 May 18;12:28. doi: 10.1186/1477-5956-12-28. eCollection 2014. Proteome Sci. 2014. PMID: 25018669 Free PMC article.
References
-
- Decker GL, Lennarz WJ. Skeletogenesis in the sea urchin embryo. Development. 1988;103:231–247. - PubMed
-
- Wilt FH. In: Handbook of Biomineralization. Bäuerlein E, editor. Vol. 1. Weinheim: Wiley-VCH; 2007. The morphogenesis and biomineralization of the sea urchin larval skeleton; pp. 183–210.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

