Dynamic transport and localization of alpha-synuclein in primary hippocampal neurons
- PMID: 20181133
- PMCID: PMC2830200
- DOI: 10.1186/1750-1326-5-9
Dynamic transport and localization of alpha-synuclein in primary hippocampal neurons
Abstract
Background: Alpha-synuclein is a presynaptic protein with a proposed role in neurotransmission and dopamine homeostasis. Abnormal accumulation of alpha-synuclein aggregates in dopaminergic neurons of the substantia nigra is diagnostic of sporadic Parkinson's disease, and mutations in the protein are linked to early onset forms of the disease. The folded conformation of the protein varies depending upon its environment and other factors that are poorly understood. When bound to phospholipid membranes, alpha-synuclein adopts a helical conformation that mediates specific interactions with other proteins.
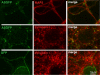
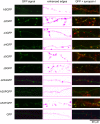
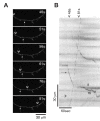
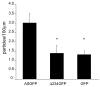
Results: To investigate the role of the helical domain in transport and localization of alpha-synuclein, eGFP-tagged constructs were transfected into rat primary hippocampal neurons at 7 DIV. A series of constructs were analyzed in which each individual exon was deleted, for comparison to previous studies of lipid affinity and alpha-helix content. A53T and A30P substitutions, representing Parkinson's disease-associated variants, were analyzed as well. Single exon deletions within the lipid-binding N-terminal domain of alpha-synuclein (exons 2, 3, and 4) partially disrupted its presynaptic localization at 17-21 DIV, resulting in increased diffuse labeling of axons. Similar results were obtained for A30P, which exhibits decreased lipid binding, but not A53T. To examine whether differences in presynaptic enrichment were related to deficiencies in transport velocity, transport was visualized via live cell microscopy. Tagged alpha-synuclein migrated at a rate of 1.85 +/- 0.09 mum/s, consistent with previous reports, and single exon deletion mutants migrated at similar rates, as did A30P. Deletion of the entire N-terminal lipid-binding domain (Delta234GFP) did not significantly alter rates of particle movement, but decreased the number of moving particles. Only the A53TGFP mutant exhibited a significant decrease in transport velocity as compared to ASGFP.
Conclusions: These results support the hypothesis that presynaptic localization involves a mechanism that requires helical conformation and lipid binding. Conversely, the rate of axonal transport is not determined by lipid affinity and is not sufficient to account for differences in presynaptic localization of alpha-synuclein-eGFP variants.
Figures

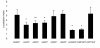

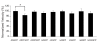
Similar articles
-
Trypsin disrupts the trafficking of the human dopamine transporter by alpha-synuclein and its A30P mutant.Biochemistry. 2004 Feb 10;43(5):1242-53. doi: 10.1021/bi035308s. Biochemistry. 2004. PMID: 14756560
-
Mutations in the lipid-binding domain of alpha-synuclein confer overlapping, yet distinct, functional properties in the regulation of dopamine transporter activity.Mol Cell Neurosci. 2003 Sep;24(1):91-105. doi: 10.1016/s1044-7431(03)00124-6. Mol Cell Neurosci. 2003. PMID: 14550771
-
Effect of familial Parkinson's disease point mutations A30P and A53T on the structural properties, aggregation, and fibrillation of human alpha-synuclein.Biochemistry. 2001 Sep 25;40(38):11604-13. doi: 10.1021/bi010616g. Biochemistry. 2001. PMID: 11560511
-
Parkinson's disease and alpha synuclein: is Parkinson's disease a prion-like disorder?Mov Disord. 2013 Jan;28(1):31-40. doi: 10.1002/mds.25373. Mov Disord. 2013. PMID: 23390095 Review.
-
Impaired dopamine storage resulting from alpha-synuclein mutations may contribute to the pathogenesis of Parkinson's disease.Hum Mol Genet. 2002 Oct 1;11(20):2395-407. doi: 10.1093/hmg/11.20.2395. Hum Mol Genet. 2002. PMID: 12351575 Review.
Cited by
-
Alpha-Synuclein affects neurite morphology, autophagy, vesicle transport and axonal degeneration in CNS neurons.Cell Death Dis. 2015 Jul 9;6(7):e1811. doi: 10.1038/cddis.2015.169. Cell Death Dis. 2015. PMID: 26158517 Free PMC article.
-
Intracellular A53T Mutant α-Synuclein Impairs Adult Hippocampal Newborn Neuron Integration.Front Cell Dev Biol. 2020 Nov 11;8:561963. doi: 10.3389/fcell.2020.561963. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33262984 Free PMC article.
-
The parkinsonian mimetic, MPP+, specifically impairs mitochondrial transport in dopamine axons.J Neurosci. 2011 May 11;31(19):7212-21. doi: 10.1523/JNEUROSCI.0711-11.2011. J Neurosci. 2011. PMID: 21562285 Free PMC article.
-
Degradation of the electrospun silica nanofiber in a biological medium for primary hippocampal neuron - effect of surface modification.Int J Nanomedicine. 2016 Feb 26;11:729-41. doi: 10.2147/IJN.S93651. eCollection 2016. Int J Nanomedicine. 2016. PMID: 27013873 Free PMC article.
-
Evidence for Immune Response, Axonal Dysfunction and Reduced Endocytosis in the Substantia Nigra in Early Stage Parkinson's Disease.PLoS One. 2015 Jun 18;10(6):e0128651. doi: 10.1371/journal.pone.0128651. eCollection 2015. PLoS One. 2015. PMID: 26087293 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

