Comparative kinetic analysis of two fungal beta-glucosidases
- PMID: 20181208
- PMCID: PMC2847552
- DOI: 10.1186/1754-6834-3-3
Comparative kinetic analysis of two fungal beta-glucosidases
Abstract
Background: The enzymatic hydrolysis of cellulose is still considered as one of the main limiting steps of the biological production of biofuels from lignocellulosic biomass. It is a complex multistep process, and various kinetic models have been proposed. The cellulase enzymatic cocktail secreted by Trichoderma reesei has been intensively investigated. beta-glucosidases are one of a number of cellulolytic enzymes, and catalyze the last step releasing glucose from the inhibitory cellobiose. beta-glucosidase (BGL1) is very poorly secreted by Trichoderma reesei strains, and complete hydrolysis of cellulose often requires supplementation with a commercial beta-glucosidase preparation such as that from Aspergillus niger (Novozymes SP188). Surprisingly, kinetic modeling of beta-glucosidases lacks reliable data, and the possible differences between native T. reesei and supplemented beta-glucosidases are not taken into consideration, possibly because of the difficulty of purifying BGL1.
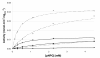
Results: A comparative kinetic analysis of beta-glucosidase from Aspergillus niger and BGL1 from Trichoderma reesei, purified using a new and efficient fast protein liquid chromatography protocol, was performed. This purification is characterized by two major steps, including the adsorption of the major cellulases onto crystalline cellulose, and a final purification factor of 53. Quantitative analysis of the resulting beta-glucosidase fraction from T. reesei showed it to be 95% pure. Kinetic parameters were determined using cellobiose and a chromogenic artificial substrate. A new method allowing easy and rapid determination of the kinetic parameters was also developed. beta-Glucosidase SP188 (Km = 0.57 mM; Kp = 2.70 mM) has a lower specific activity than BGL1 (Km = 0.38 mM; Kp = 3.25 mM) and is also more sensitive to glucose inhibition. A Michaelis-Menten model integrating competitive inhibition by the product (glucose) has been validated and is able to predict the beta-glucosidase activity of both enzymes.
Conclusions: This article provides a useful comparison between the activity of beta-glucosidases from two different fungi, and shows the importance of fully characterizing both enzymes. A Michaelis-Menten model was developed, including glucose inhibition and kinetic parameters, which were accurately determined and compared. This model can be further integrated into a cellulose hydrolysis model dissociating beta-glucosidase activity from that of other cellulases. It can also help to define the optimal enzymatic cocktails for new beta-glucosidase activities.
Figures



Similar articles
-
Physiochemical and Thermodynamic Characterization of Highly Active Mutated Aspergillus niger β-glucosidase for Lignocellulose Hydrolysis.Protein Pept Lett. 2018;25(2):208-219. doi: 10.2174/0929866525666180130161504. Protein Pept Lett. 2018. PMID: 29384047
-
Heterologously expressed Aspergillus aculeatus β-glucosidase in Saccharomyces cerevisiae is a cost-effective alternative to commercial supplementation of β-glucosidase in industrial ethanol production using Trichoderma reesei cellulases.J Biosci Bioeng. 2016 Jan;121(1):27-35. doi: 10.1016/j.jbiosc.2015.05.002. J Biosci Bioeng. 2016. PMID: 26073313
-
Dissecting Cellular Function and Distribution of β-Glucosidases in Trichoderma reesei.mBio. 2021 May 11;12(3):e03671-20. doi: 10.1128/mBio.03671-20. mBio. 2021. PMID: 33975944 Free PMC article.
-
Genetic modification: A tool for enhancing beta-glucosidase production for biofuel application.Bioresour Technol. 2017 Dec;245(Pt B):1352-1361. doi: 10.1016/j.biortech.2017.05.126. Epub 2017 May 21. Bioresour Technol. 2017. Retraction in: Bioresour Technol. 2025 Jan;415:131674. doi: 10.1016/j.biortech.2024.131674. PMID: 28596076 Retracted. Review.
-
Bioconversion of lignocellulosic biomass: biochemical and molecular perspectives.J Ind Microbiol Biotechnol. 2008 May;35(5):377-391. doi: 10.1007/s10295-008-0327-8. Epub 2008 Mar 13. J Ind Microbiol Biotechnol. 2008. PMID: 18338189 Review.
Cited by
-
A review of the enzymatic hydrolysis of mannans and synergistic interactions between β-mannanase, β-mannosidase and α-galactosidase.World J Microbiol Biotechnol. 2015 Aug;31(8):1167-75. doi: 10.1007/s11274-015-1878-2. Epub 2015 May 31. World J Microbiol Biotechnol. 2015. PMID: 26026279 Review.
-
Functional diversity of family 3 β-glucosidases from thermophilic cellulolytic fungus Humicola insolens Y1.Sci Rep. 2016 Jun 8;6:27062. doi: 10.1038/srep27062. Sci Rep. 2016. PMID: 27271847 Free PMC article.
-
Novel Ethanol- and 5-Hydroxymethyl Furfural-Stimulated β-Glucosidase Retrieved From a Brazilian Secondary Atlantic Forest Soil Metagenome.Front Microbiol. 2018 Oct 29;9:2556. doi: 10.3389/fmicb.2018.02556. eCollection 2018. Front Microbiol. 2018. PMID: 30420843 Free PMC article.
-
Mucoromycota fungi as powerful cell factories for modern biorefinery.Appl Microbiol Biotechnol. 2022 Jan;106(1):101-115. doi: 10.1007/s00253-021-11720-1. Epub 2021 Dec 10. Appl Microbiol Biotechnol. 2022. PMID: 34889982 Review.
-
Enhanced Bioconversion of Cellobiose by Industrial Saccharomyces cerevisiae Used for Cellulose Utilization.Front Microbiol. 2016 Mar 3;7:241. doi: 10.3389/fmicb.2016.00241. eCollection 2016. Front Microbiol. 2016. PMID: 26973619 Free PMC article.
References
-
- Reese ET, Mandels M. In: Cellulose and Cellulose Derivatives. Bikales NM, Segal L, editor. New York: Wiley Interscience; 1971. Enzymatic degradation; pp. 1079–1094.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

