Inhaled salmeterol and/or fluticasone alters structure/function in a murine model of allergic airways disease
- PMID: 20181256
- PMCID: PMC2841146
- DOI: 10.1186/1465-9921-11-22
Inhaled salmeterol and/or fluticasone alters structure/function in a murine model of allergic airways disease
Abstract
Background: The relationship between airway structural changes (remodeling) and airways hyperresponsiveness (AHR) is unclear. Asthma guidelines suggest treating persistent asthma with inhaled corticosteroids and long acting beta-agonists (LABA). We examined the link between physiological function and structural changes following treatment fluticasone and salmeterol separately or in combination in a mouse model of allergic asthma.
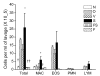
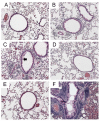
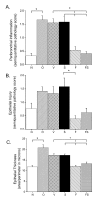
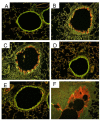
Methods: BALB/c mice were sensitized to intraperitoneal ovalbumin (OVA) followed by six daily inhalation exposures. Treatments included 9 daily nebulized administrations of fluticasone alone (6 mg/ml), salmeterol (3 mg/ml), or the combination fluticasone and salmeterol. Lung impedance was measured following methacholine inhalation challenge. Airway inflammation, epithelial injury, mucus containing cells, and collagen content were assessed 48 hours after OVA challenge. Lungs were imaged using micro-CT.
Results and discussion: Treatment of allergic airways disease with fluticasone alone or in combination with salmeterol reduced AHR to approximately naüve levels while salmeterol alone increased elastance by 39% compared to control. Fluticasone alone and fluticasone in combination with salmeterol both reduced inflammation to near naive levels. Mucin containing cells were also reduced with fluticasone and fluticasone in combination with salmeterol.
Conclusions: Fluticasone alone and in combination with salmeterol reduces airway inflammation and remodeling, but salmeterol alone worsens AHR: and these functional changes are consistent with the concomitant changes in mucus metaplasia.
Figures


Similar articles
-
Treatment comparison of budesonide/formoterol with salmeterol/fluticasone propionate in adults aged > or =16 years with asthma: post hoc analysis of a randomized, double-blind study.Clin Drug Investig. 2010;30(9):565-79. doi: 10.2165/11533450-000000000-00000. Clin Drug Investig. 2010. PMID: 20593912 Clinical Trial.
-
Combined fluticasone propionate and salmeterol reduces RSV infection more effectively than either of them alone in allergen-sensitized mice.Virol J. 2006 May 23;3:32. doi: 10.1186/1743-422X-3-32. Virol J. 2006. PMID: 16719922 Free PMC article.
-
Fluticasone/formoterol combination therapy is as effective as fluticasone/salmeterol in the treatment of asthma, but has a more rapid onset of action: an open-label, randomized study.BMC Pulm Med. 2011 May 23;11:28. doi: 10.1186/1471-2466-11-28. BMC Pulm Med. 2011. PMID: 21605396 Free PMC article. Clinical Trial.
-
Comparing the efficacy and safety of salmeterol/fluticasone pMDI versus its mono-components, other LABA/ICS pMDIs and salmeterol/fluticasone Diskus in patients with asthma.Expert Opin Drug Deliv. 2015 Jun;12(6):963-75. doi: 10.1517/17425247.2015.987661. Epub 2014 Nov 27. Expert Opin Drug Deliv. 2015. PMID: 25429610 Review.
-
Inhaled salmeterol/fluticasone propionate: a review of its use in asthma.Drugs. 2005;65(12):1715-34. doi: 10.2165/00003495-200565120-00012. Drugs. 2005. PMID: 16060707 Review.
Cited by
-
Use of a novel one-nostril mask-spacer device to evaluate airway hyperresponsiveness (AHR) in horses after chronic administration of albuterol.Can J Vet Res. 2014 Jul;78(3):214-20. Can J Vet Res. 2014. PMID: 24982553 Free PMC article. Clinical Trial.
-
Co-inhalation of roflumilast, rather than formoterol, with fluticasone more effectively improves asthma in asthmatic mice.Exp Biol Med (Maywood). 2017 Mar;242(5):516-526. doi: 10.1177/1535370216685006. Epub 2017 Jan 5. Exp Biol Med (Maywood). 2017. PMID: 28056550 Free PMC article.
-
Magnetic Nanodrug Delivery Through the Mucus Layer of Air-Liquid Interface Cultured Primary Normal Human Tracheobronchial Epithelial Cells.Bionanoscience. 2016 Sep;6(3):235-242. doi: 10.1007/s12668-016-0216-y. Epub 2016 Jul 28. Bionanoscience. 2016. PMID: 27774374 Free PMC article.
-
Potentiated interaction between ineffective doses of budesonide and formoterol to control the inhaled cadmium-induced up-regulation of metalloproteinases and acute pulmonary inflammation in rats.PLoS One. 2014 Oct 14;9(10):e109136. doi: 10.1371/journal.pone.0109136. eCollection 2014. PLoS One. 2014. PMID: 25313925 Free PMC article.
-
Role for β-arrestin in mediating paradoxical β2AR and PAR2 signaling in asthma.Curr Opin Pharmacol. 2014 Jun;16:142-7. doi: 10.1016/j.coph.2014.03.007. Epub 2014 Jun 5. Curr Opin Pharmacol. 2014. PMID: 24907413 Free PMC article. Review.
References
-
- McParland BE, Macklem PT, Pare PD. Airway wall remodeling: friend or foe? J Appl Physiol. 2003;95:426–434. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

