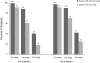First-line latanoprost therapy in ocular hypertension or open-angle glaucoma patients: a 3-month efficacy analysis stratified by initial intraocular pressure
- PMID: 20181282
- PMCID: PMC2841111
- DOI: 10.1186/1471-2415-10-4
First-line latanoprost therapy in ocular hypertension or open-angle glaucoma patients: a 3-month efficacy analysis stratified by initial intraocular pressure
Abstract
Background: Prospective, multicenter, randomized, double-masked trials have shown latanoprost instilled once daily to be at least as effective as and generally superior to timolol administered twice daily and to be as effective as other frequently prescribed prostaglandin analogues. This study prospectively assessed the efficacy of latanoprost monotherapy in a large cohort of treatment-naive patients with a broad range of baseline intraocular pressure (IOP) levels treated in actual clinical practice settings.
Methods: This prospective, open-label, multicenter, uncontrolled, phase IV study included treatment-naive ocular hypertension or open-angle glaucoma subjects initiating latanoprost once daily (evening). IOP levels were measured at baseline and after 1 and 3 months. The primary efficacy outcome was mean change in IOP from baseline to month 3. Analyses were stratified by baseline IOP: > or = 20 and <24 mmHg vs > or = 24 mmHg.
Results: Efficacy analyses (intent to treat) included 572 subjects: 20 to <24 mmHg group, N = 252; > or = 24 mmHg group, N = 320. Mean baseline IOP levels were 22.2 +/- 0.9 mmHg and 26.7 +/- 2.8 mmHg, respectively. At month 3, significant IOP reductions were seen in both groups (p < 0.0001, within-group differences); reductions were smaller in the 20 to <24 mmHg group (-6.3 +/- 2.4 vs -9.2 +/- 3.7 mmHg, respectively; -28.0 +/- 10.6% vs -34.1 +/- 11.9%, respectively). An IOP reduction of > or = 30% from baseline to month 3 was noted in 48.4% and 65.6% of subjects, respectively (p < 0.0001). At month 3, targets IOPs of < or = 18 mmHg were achieved by > or = 70% of subjects in both groups. Latanoprost was well tolerated with an adverse event profile similar to that reported in the literature.
Conclusions: This "real world" study found once-daily latanoprost to be effective and safe in treatment-naive ocular hypertension or open-angle glaucoma patients. Patients with baseline IOP levels of 20 to <24 mmHg as well as > or = 24 mmHg benefitted from initial latanoprost therapy.
Trial registration number: NCT00647101.
Figures
Similar articles
-
The additive intraocular pressure-lowering effect of latanoprost 0.005% daily once and pilocarpine 2% t.i.d. in patients with open-angle glaucoma or ocular hypertension. a 6-month, randomized, multicenter study. German Latanoprost Study Group.Graefes Arch Clin Exp Ophthalmol. 2000 May;238(5):433-9. doi: 10.1007/s004170050375. Graefes Arch Clin Exp Ophthalmol. 2000. PMID: 10901475 Clinical Trial.
-
A 1-year study to compare the efficacy and safety of once-daily travoprost 0.004%/timolol 0.5% to once-daily latanoprost 0.005%/timolol 0.5% in patients with open-angle glaucoma or ocular hypertension.Eur J Ophthalmol. 2007 Mar-Apr;17(2):183-90. doi: 10.1177/112067210701700206. Eur J Ophthalmol. 2007. PMID: 17415690 Clinical Trial.
-
A 4-week, dose-ranging study comparing the efficacy, safety and tolerability of latanoprost 75, 100 and 125 μg/mL to latanoprost 50 μg/mL (xalatan) in the treatment of primary open-angle glaucoma and ocular hypertension.BMC Ophthalmol. 2012 May 18;12:9. doi: 10.1186/1471-2415-12-9. BMC Ophthalmol. 2012. PMID: 22607109 Free PMC article. Clinical Trial.
-
Latanoprost : an update of its use in glaucoma and ocular hypertension.Drugs Aging. 2003;20(8):597-630. doi: 10.2165/00002512-200320080-00005. Drugs Aging. 2003. PMID: 12795627 Review.
-
Human experience and efficacy of omidenepag isopropyl (Eybelis®; Omlonti®): Discovery to approval of the novel non-prostaglandin EP2-receptor-selective agonist ocular hypotensive drug.Curr Opin Pharmacol. 2024 Feb;74:102426. doi: 10.1016/j.coph.2023.102426. Epub 2024 Jan 1. Curr Opin Pharmacol. 2024. PMID: 38168596 Review.
Cited by
-
Efficacy and safety of tafluprost in normal-tension glaucoma with intraocular pressure of 16 mmHg or less.Jpn J Ophthalmol. 2011 Nov;55(6):605-13. doi: 10.1007/s10384-011-0082-7. Epub 2011 Aug 27. Jpn J Ophthalmol. 2011. PMID: 21874307
-
Current primary open-angle glaucoma treatments and future directions.Clin Ophthalmol. 2012;6:1699-707. doi: 10.2147/OPTH.S32933. Epub 2012 Oct 23. Clin Ophthalmol. 2012. PMID: 23118520 Free PMC article.
-
Preservative-free tafluprost in the treatment of naive patients with glaucoma and ocular hypertension.Clin Ophthalmol. 2013;7:901-10. doi: 10.2147/OPTH.S41640. Epub 2013 May 16. Clin Ophthalmol. 2013. PMID: 23717036 Free PMC article.
-
Stanniocalcin-1 Reduced Intraocular Pressure in Two Models of Ocular Hypertension.Curr Eye Res. 2021 Oct;46(10):1525-1530. doi: 10.1080/02713683.2021.1899246. Epub 2021 Mar 24. Curr Eye Res. 2021. PMID: 33757401 Free PMC article.
-
Retrospective Chart Review on Real-World Use of Latanoprostene Bunod 0.024% in Treatment-Naïve Patients with Open-Angle Glaucoma.Ophthalmol Ther. 2020 Dec;9(4):1041-1053. doi: 10.1007/s40123-020-00307-0. Epub 2020 Oct 7. Ophthalmol Ther. 2020. PMID: 33034885 Free PMC article.
References
-
- Friedman DS, Wilson MR, Liebmann JM, Fechtner RD, Weinreb RN. An evidence-based assessment of risk factors for the progression of ocular hypertension and glaucoma. Am J Ophthalmol. 2004;138(3 Suppl):S19–31. - PubMed
-
- Kass MA, Gordon M, Morley RE Jr, Meltzer DW, Goldberg JJ. Compliance with topical timolol treatment. Am J Ophthalmol. 1987;103:188–193. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical