Histopathology and apoptosis in an animal model of reversible renal injury
- PMID: 20181466
- PMCID: PMC3951867
- DOI: 10.1016/j.etp.2010.02.002
Histopathology and apoptosis in an animal model of reversible renal injury
Abstract
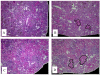
High adenine phosphate (HAP) diet serves as an animal model of chronic renal failure (RF). Induction of RF and establishment of end organ damage require long exposure periods to this diet. Previously, we have shown that RF is reversible after diet cessation even after protracted administration. In this study, we explored the underlying renal changes and cellular pathways occurring during administration and after cessation of the diet. Kidneys were obtained from rats fed HAP diet for 7 weeks, and from rats fed HAP diet followed a 10 week recovery period on normal diet. The kidneys of HAP diet group were significantly enlarged due to tubular injury characterized by massive cystic dilatation and crystal deposition. Kidney injury was associated with markers of apoptosis as well as with activation of apoptosis related pathways. Diet cessation was associated with a significant reduction in kidney size, tubules diameter, and crystals deposition. The recovery from renal injury was coupled with regression of apoptotic features. This is the first study showing the potential reversibility of long standing RF model, allowing optimal evaluation of uremia-chronic effects.
Copyright © 2010 Elsevier GmbH. All rights reserved.
Figures


References
-
- Bellomo R, Ronco C, Kellum J, Mehta R, Palevsky P, The ADQI workgroup Acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Critical Care. 2004;8:R204–12. - PMC - PubMed
-
- Ben-Sasson S, Sherman Y, Gavrieli Y. Identification of dying cells – in situ staining. Methods Cell Biol. 1995;46:29–39. - PubMed
-
- Go A, Chertow GM, Fan D, McCulloch CE, Hsu C. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–305. - PubMed
-
- Heyman SN, Rosen S, Darmon D, Goldfarb M, Bitz H, Shina A, et al. Endotoxin-induced renal failure: II. A role for tubular hypoxic damage. Exp Nephrol. 2000;8:275–82. - PubMed
-
- Katsumata K, Kenichiro K, Michinori H, Kunihiko T, Nobuo N, Steven KB, et al. Sevelamer hydrochloride prevents ectopic calcification and renal osteodystrophy in chronic renal failure rats. Kidney Int. 2003;64:441–50. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

