Critical involvement of postsynaptic protein kinase activation in long-term potentiation at hippocampal mossy fiber synapses on CA3 interneurons
- PMID: 20181582
- PMCID: PMC2854556
- DOI: 10.1523/JNEUROSCI.5269-09.2010
Critical involvement of postsynaptic protein kinase activation in long-term potentiation at hippocampal mossy fiber synapses on CA3 interneurons
Abstract
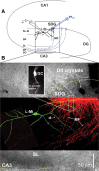
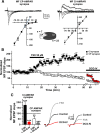
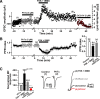
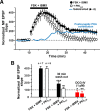
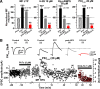
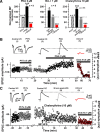
Hippocampal mossy fiber (MF) synapses on area CA3 lacunosum-moleculare (L-M) interneurons are capable of undergoing a Hebbian form of NMDA receptor (NMDAR)-independent long-term potentiation (LTP) induced by the same type of high-frequency stimulation (HFS) that induces LTP at MF synapses on pyramidal cells. LTP of MF input to L-M interneurons occurs only at synapses containing mostly calcium-impermeable (CI)-AMPA receptors (AMPARs). Here, we demonstrate that HFS-induced LTP at these MF-interneuron synapses requires postsynaptic activation of protein kinase A (PKA) and protein kinase C (PKC). Brief extracellular stimulation of PKA with forskolin (FSK) alone or in combination with 1-Methyl-3-isobutylxanthine (IBMX) induced a long-lasting synaptic enhancement at MF synapses predominantly containing CI-AMPARs. However, the FSK/IBMX-induced potentiation in cells loaded with the specific PKA inhibitor peptide PKI(6-22) failed to be maintained. Consistent with these data, delivery of HFS to MFs synapsing onto L-M interneurons loaded with PKI(6-22) induced posttetanic potentiation (PTP) but not LTP. Hippocampal sections stained for the catalytic subunit of PKA revealed abundant immunoreactivity in interneurons located in strata radiatum and L-M of area CA3. We also found that extracellular activation of PKC with phorbol 12,13-diacetate induced a pharmacological potentiation of the isolated CI-AMPAR component of the MF EPSP. However, HFS delivered to MF synapses on cells loaded with the PKC inhibitor chelerythrine exhibited PTP followed by a significant depression. Together, our data indicate that MF LTP in L-M interneurons at synapses containing primarily CI-AMPARs requires some of the same signaling cascades as does LTP of glutamatergic input to CA3 or CA1 pyramidal cells.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
