Reconciling coherent oscillation with modulation of irregular spiking activity in selective attention: gamma-range synchronization between sensory and executive cortical areas
- PMID: 20181583
- PMCID: PMC2888157
- DOI: 10.1523/JNEUROSCI.4222-09.2010
Reconciling coherent oscillation with modulation of irregular spiking activity in selective attention: gamma-range synchronization between sensory and executive cortical areas
Abstract
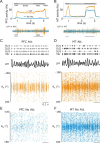
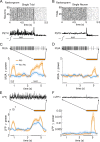
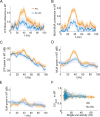
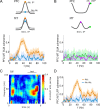
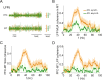
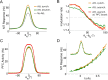
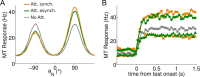
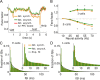
In this computational work, we investigated gamma-band synchronization across cortical circuits associated with selective attention. The model explicitly instantiates a reciprocally connected loop of spiking neurons between a sensory-type (area MT) and an executive-type (prefrontal/parietal) cortical circuit (the source area for top-down attentional signaling). Moreover, unlike models in which neurons behave as clock-like oscillators, in our model single-cell firing is highly irregular (close to Poisson), while local field potential exhibits a population rhythm. In this "sparsely synchronized oscillation" regime, the model reproduces and clarifies multiple observations from behaving animals. Top-down attentional inputs have a profound effect on network oscillatory dynamics while only modestly affecting single-neuron spiking statistics. In addition, attentional synchrony modulations are highly selective: interareal neuronal coherence occurs only when there is a close match between the preferred feature of neurons, the attended feature, and the presented stimulus, a prediction that is experimentally testable. When interareal coherence was abolished, attention-induced gain modulations of sensory neurons were slightly reduced. Therefore, our model reconciles the rate and synchronization effects, and suggests that interareal coherence contributes to large-scale neuronal computation in the brain through modest enhancement of rate modulations as well as a pronounced attention-specific enhancement of neural synchrony.
Figures


Similar articles
-
Inhibitory synchrony as a mechanism for attentional gain modulation.J Physiol Paris. 2004 Jul-Nov;98(4-6):296-314. doi: 10.1016/j.jphysparis.2005.09.002. Epub 2005 Nov 7. J Physiol Paris. 2004. PMID: 16274973 Free PMC article.
-
Attention Configures Synchronization Within Local Neuronal Networks for Processing of the Behaviorally Relevant Stimulus.Front Neural Circuits. 2018 Aug 29;12:71. doi: 10.3389/fncir.2018.00071. eCollection 2018. Front Neural Circuits. 2018. PMID: 30210309 Free PMC article.
-
Neuronal synchronization along the dorsal visual pathway reflects the focus of spatial attention.Neuron. 2008 Nov 26;60(4):709-19. doi: 10.1016/j.neuron.2008.09.010. Neuron. 2008. PMID: 19038226
-
Cortical up and activated states: implications for sensory information processing.Neuroscientist. 2009 Dec;15(6):625-34. doi: 10.1177/1073858409333074. Neuroscientist. 2009. PMID: 19321459 Free PMC article. Review.
-
Cortical oscillatory activity and the dynamics of auditory memory processing.Rev Neurosci. 2005;16(3):239-54. doi: 10.1515/revneuro.2005.16.3.239. Rev Neurosci. 2005. PMID: 16323563 Review.
Cited by
-
Transcranial alternating current stimulation (tACS) at gamma frequency: an up-and-coming tool to modify the progression of Alzheimer's Disease.Transl Neurodegener. 2024 Jun 27;13(1):33. doi: 10.1186/s40035-024-00423-y. Transl Neurodegener. 2024. PMID: 38926897 Free PMC article. Review.
-
Regulation of Irregular Neuronal Firing by Autaptic Transmission.Sci Rep. 2016 May 17;6:26096. doi: 10.1038/srep26096. Sci Rep. 2016. PMID: 27185280 Free PMC article.
-
Across-Area Synchronization Supports Feature Integration in a Biophysical Network Model of Working Memory.Front Neural Circuits. 2021 Sep 20;15:716965. doi: 10.3389/fncir.2021.716965. eCollection 2021. Front Neural Circuits. 2021. PMID: 34616279 Free PMC article.
-
Higher dopamine D1 receptor expression in prefrontal parvalbumin neurons underlies higher distractibility in marmosets versus macaques.Commun Biol. 2025 Jul 1;8(1):974. doi: 10.1038/s42003-025-08297-0. Commun Biol. 2025. PMID: 40594842 Free PMC article.
-
Mechanisms of gamma oscillations.Annu Rev Neurosci. 2012;35:203-25. doi: 10.1146/annurev-neuro-062111-150444. Epub 2012 Mar 20. Annu Rev Neurosci. 2012. PMID: 22443509 Free PMC article. Review.
References
-
- Albright TD. Direction and orientation selectivity of neurons in visual area MT of the macaque. J Neurophysiol. 1984;52:1106–1130. - PubMed
-
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. - PubMed
-
- Barbas H. Anatomic organization of basoventral and mediodorsal visual recipient prefrontal regions in the rhesus monkey. J Comp Neurol. 1988;276:313–342. - PubMed
