Lack of Mid1, the mouse ortholog of the Opitz syndrome gene, causes abnormal development of the anterior cerebellar vermis
- PMID: 20181585
- PMCID: PMC6633954
- DOI: 10.1523/JNEUROSCI.4196-09.2010
Lack of Mid1, the mouse ortholog of the Opitz syndrome gene, causes abnormal development of the anterior cerebellar vermis
Abstract
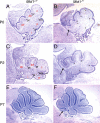
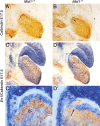
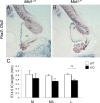
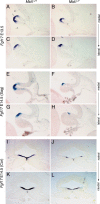
Opitz G/BBB syndrome (OS) is a genetic disorder characterized by midline developmental defects. Male patients with the X-linked form of OS, caused by loss-of-function mutations in the MID1 gene, show high variability of the clinical signs. MID1 encodes a ubiquitin ligase that controls phosphatase 2A, but its role in the pathogenesis of the disease is still unclear. Here, we report a mouse line carrying a nonfunctional ortholog of the human MID1 gene, Mid1. Mid1-null mice show the brain anatomical defect observed in patients (i.e., hypoplasia of the anterior portion of the medial cerebellum, the vermis). We found that the presence of this defect correlates with motor coordination and procedural and nonassociative learning impairments. The defect is limited to the most anterior lobes of the vermis, the region of the developing cerebellum adjacent to the dorsal midbrain. Analyses at midgestation reveal that lack of Mid1 causes the shortening of the posterior dorsal midbrain, the rostralization of the midbrain/cerebellum boundary, and the downregulation of a key player in the development of this region, Fgf17. Thus, lack of Mid1 causes a misspecification of the midbrain/cerebellar boundary that results in an abnormal development of the most anterior cerebellar lobes. This animal model provides a tool for additional in vivo studies of the physiological and pathological role of the Mid1 gene and a system to investigate the development and function of anterior cerebellar domains.
Figures



References
-
- Bobée S, Mariette E, Tremblay-Leveau H, Caston J. Effects of early midline cerebellar lesion on cognitive and emotional functions in the rat. Behav Brain Res. 2000;112:107–117. - PubMed
-
- Broccoli V, Boncinelli E, Wurst W. The caudal limit of Otx2 expression positions the isthmic organizer. Nature. 1999;401:164–168. - PubMed
-
- Buchner G, Montini E, Andolfi G, Quaderi N, Cainarca S, Messali S, Bassi MT, Ballabio A, Meroni G, Franco B. MID2, a homologue of the Opitz syndrome gene MID1: similarities in subcellular localization and differences in expression during development. Hum Mol Genet. 1999;8:1397–1407. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
